
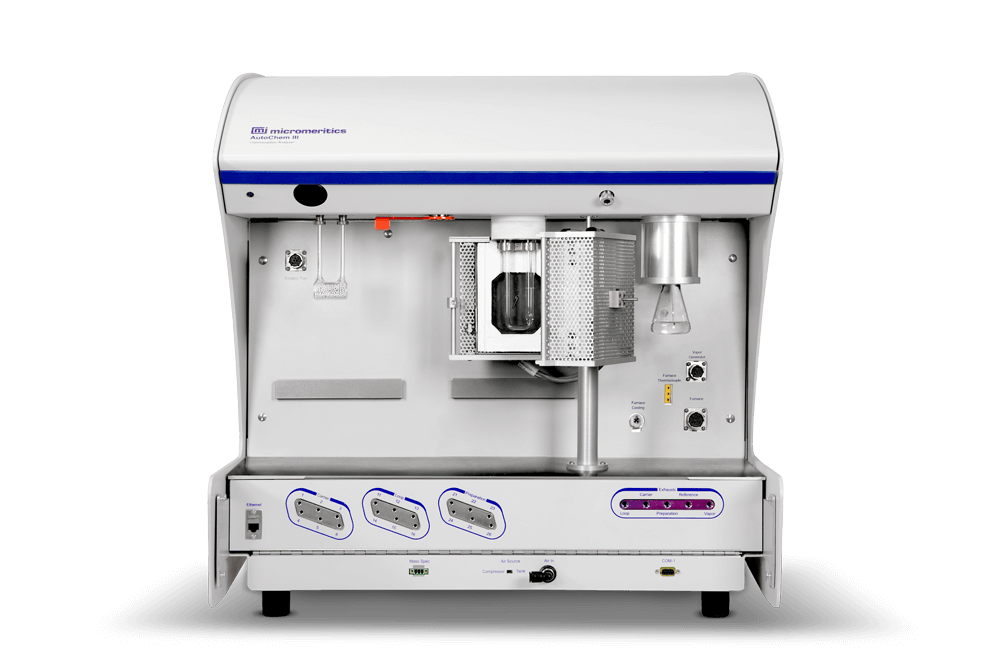
The AutoChem from Micromeritics is the most widely used and highly cited system for catalyst reactivity characterization because it is also the most automated, highly accurate system for chemisorption and temperature-programmed reactions.
The all new AutoChem III meets and exceeds that performance with a design that will save you hours a day, make the most sensitive, reproducible measurement, and enhance operator safety.
provides superior moisture removal for TPR experiments with a system that is effortless to use and saves hours per day
Internal gas temperature control
Lowest internal gas volume
High sensitivity thermal
conductivity detector (TCD)
Temperature-controlled
corrosion-resistant detector
Exclusive AutoTrap
KwikConnect
Dynamic clamshell furnace
Integrated AutoCool
18 total gas inlets
The new AutoChem III is designed to make key operations quick and easy, saving you hours a day so you can spend less time making measurements and more time making progress.
The NEW AutoCool is an integrated gas-fed system that rapidly cools sample tubes before and during experiments. AutoCool is typically 30 minutes faster than alternative systems and requires no liquids or external support. The NEW AutoTrap effectively traps vapor and requires no manual slush bath preparation.
Exclusive automated detector calibration
The AutoChem III makes quantitative accuracy simple with automated detector calibration. Traditional systems require calibration by multiple runs of reference materials or single-point offsets that ignore changes in temperature or pressure.
The AutoChem III generates accurate results through a fully automated process using the system’s patented gas blending capabilities including compensation for injection loop temperature and pressure to ensure the highest calibration – and result – accuracy. The process is fast, automated, requires no operator intervention, and produces more accurate results than alternative designs.
The patented new KwikConnect makes sample tube installation faster, easier, and more reliable than traditional designs with half as many separate pieces and no threaded fittings. Installation and removal are easier and quicker, reduce the risk of breaking sample tubes, and provide peace of mind that the snap lock closure has completely sealed the system.
Don’t waste time reconnecting and switching gas lines: have what you need ready when you need it. The AutoChem III has 18 available gas streams so you’re always ready to run your next reaction. Having the right blended gas ready also means you won’t introduce errors from poorly designed external gas connections, and you won’t compromise data accuracy by blending gases, which unnecessarily introduces errors from mass flow controllers.
The AutoChem III provides results that drive confident decisions. The highest available measurement accuracy and repeatability – made under conditions that match your reaction environment – give you certainty to act with confidence.
Exacting thermal accuracy is essential to simulate reaction conditions while preventing the deactivation of precious catalyst materials. The AutoChem III exceeds all available systems in every key performance characteristic
The AutoChem III features the lowest gas flow path volume to eliminate carryover and signal tailing when changing gas flow conditions. This guarantees precise gas stream composition, even when switching configurations from one experiment to another.
And with 18 available gas inlets, you will have the gas composition that you need ready, without introducing errors associated with blending gases in situ.
FURNACE: to simulate reaction conditions
VAPOR: to control vapor composition
GAS STREAM: to maximize detection sensitivity
DETECTOR: to ensure robustness
The new AutoChem III features a new thermal conductivity detector (TCD) that is 110% more sensitive than previous designs. This enables you to use lower sample masses, accurately detect secondary reactions, and achieve greater accuracy of catalyst traits like site coverage.
Detector sensitivity is enhanced by a reference stream with a dedicated mass flow controller (MFC) that provides a stable reference to the sample stream. Alternative designs use a common carrier stream for both the reference and signal paths, resulting in interference between the measurement and reference stream leading to signal instability.
The temperature-controlled TCD is a robust sensor with a long operating life and intrinsic protection from operational errors like gas flow leaks that cause premature failure of 4-element detectors used in inferior designs.
Achieve faster analysis and more complete characterization of surface selectivity and functionality with the available vapor generator featuring automated vapor calibration, injection repeatability better than 1%, and all new continuous dosing capabilities.
This system creates uniform streams of saturated vapors such as water, alcohols, amines, or organics that are used to prepare samples for TPD or as the reaction gas stream.
The new continuous dosing capability enables faster and more uniform vapor dosing than legacy systems that are limited to discrete vapor stream pulses.
Rapidly transition from experimental data to material characteristics with Micromeritics’ own AutoChem data analysis software. Get all the answers you need with:
The AutoChem III enhances operator safety at every stage of the measurement, reducing opportunities for exposure and potential for hazardous conditions.
The new AutoTrap removes moisture without cryogenic liquids such as liquid nitrogen. The AutoTrap also eliminates the need for slush bath preparation, which requires vigorous mixing of alcohols and other solvents in glass vacuum flasks.
The new AutoCool brings sample tubes to room temperature so fast after every experiment that you can change samples and start the next experiment quickly, without handling hot glass sample tubes. And the KwikConnect sample tube retention system allows you to release the tube in one motion without fumbling with threaded connections and separate adapter pieces.
Micromeritics products are third-party-tested to conform to the highest level of regulatory compliance and operational safety. Install and run with the confidence that the system will meet or exceed requirements for electrical safety and compatibility with the need for separate qualifications or assessments.
Mass spec provides a direct probe for the identity and quantity of specific reaction products. This is especially valuable when investigating an unknown reaction, or a reaction that creates multiple products.
The single quadrupole mass spec with heated transfer line provides detection of mass fragments up to 200 amu and data collection that is integrated with the operation of the AutoChem III.
The AutoChem III also includes a general mass spec communication port for coordination with a lab’s existing mass spec.
Prepare samples for analysis or perform measurements in the presence of pulsed or continuous vapor streams such as water, alcohol, pyridine, aromatic organics, and more.
Begin experiments at temperatures as low as -100 °C with controlled liquid-nitrogen-based cooling.
For reaction chemistries that require particularly aggressive gas compositions, a special version of the AutoChem III is available with enhanced corrosion resistance. Wetted materials are constructed from highly resistant Hastelloy, highly stable perfluoroelastomers, and inert-coated stainless steel to provide the greatest stability under the harshest working conditions.
The development of efficient and effective catalysts is necessary to the continued...
+Platinum-based catalysts including Pt/C, PtRu/C, and PtRuIr/C are often characterized by temperature-programmed...
+Manganese, cobalt, bismuth, iron, copper, and silver catalysts used for the gas-phase...
+Acid catalysts such as zeolites are used to convert large hydrocarbons to...
+Catalysts containing platinum, rhenium, tin, etc. on silica, alumina, or silica alumina...
+Catalysts such as small-pore zeolites (mordenite and ZSM-5) containing noble metals (typically...
+Hydrocracking catalysts typically composed of metal sulfides (nickel, tungsten, cobalt, and molybdenum)...
+The water-gas shift reaction is an important element in the hydrogen lifecycle...
+| Temperature | Ambient to 1200°C |
| Temperature Ramp Rates | -100°C to 800°C: up to 100°C/min |
| Preparation gases | 6 inlets: H2, O2, He, Ar, H2/Ar, and more |
| Carrier gases | 6 inlets: He, Ar, H2/Ar, and more |
| Analysis (loop) gases | He, H2, CO, O2, N2O, NH3/He, and more |
Provided specifications were valid as taken from available documents at time of publication. These specifications may change without notice and are only provided as a general reference
Request a quote or talk to an expert for more information
to stay connected to product news, software updates, and the latest scientific resources
Copyright © 2025 Micromeritics Instrument Corporation