Pham Thanh Huyen, Nguyen Anh Vu, Nguyen Minh Hien, Le Van Hieu, Dao Van Tuong, Hoang Trong Yem. Petrochemical and Catalysis Material Laboratory, Hanoi University of Technology, Hanoi, Vietnam
Introduction
Since V2O5/TiO2 catalysts were successful in the oxidation of oxylene, vanadium-containing catalysts were widely used in the oxidation of other aromatic and paraffinic hydrocarbons [1]. The characterization of these catalysts has been investigated by a great number of physical and chemical methods in many laboratories. But their redox property is not without controversy. TPR (Temperature-Programmed Reduction) and NH3 TPD (Temperature-Programmed Desorption) are powerful methods to characterize the redox property and the surface acidity of solid catalysts [2,3]. In this article, the influence of different supports on the redox property and the acidity of V2O5 catalysts will be clarified by TPR and NH3 TPD.
Experimental
Catalyst Preparation
Two systems of catalysts were prepared by the wet impregnation. They were indicated as VxA (for V2O5/Al2O3) and VyT (for V2O5/TiO2), where x and y are the loadings of vanadium expressed in wt. %. Alumina (BET surface area = 195 m2/g) and Titania (BET surface area = 55 m2/g) were impregnated with vanadium oxalate aqueous solution, followed by drying at 110 °C for 14h and calcination at 650 ºC and at 450 °C (for VxA and VyT, respectively) for 3h.
Characterization of Catalysts
Temperature- Programmed Reduction (TPR) with hydrogen and Temperature-Programmed Desorption (TPD) of NH3 were carried out in a Micromeritics AutoChem II 2920 analyzer.
In the TPR experiments, the sample without pretreatment was reduced with a 10% H2/Ar mixture (25 ml/min) by heating at 10 °C/min to 800 °C. In the TPD experiments, the sample, after decontaminating at 300 °C, was saturated with 10% NH3/He (15 ml/min) at 100 °C for 1h, and then was purged with pure He for 1h. For the desorption, it was heated (10 °C/min) to 500 °C in flowing He (25 ml/min).
Results and Discussion
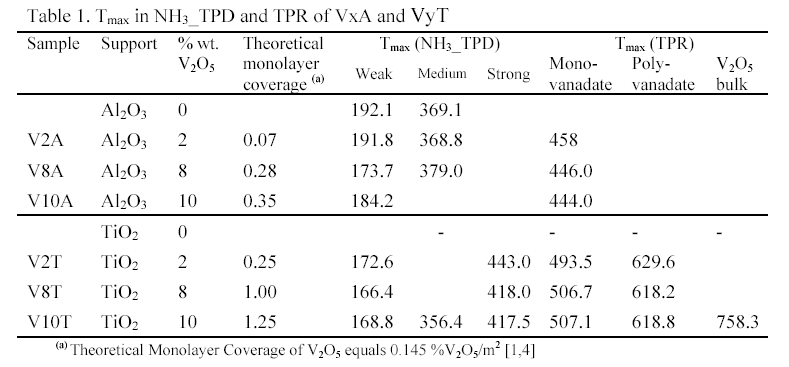
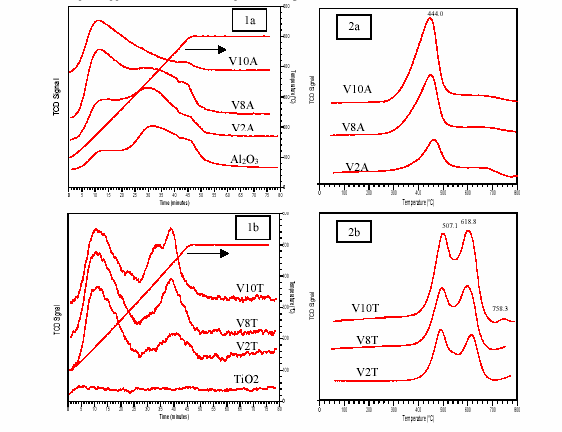
NH3 TPD of VxA contrasted with that of VyT. When the loadings of vanadia rose, the NH3 TPD profiles of VxA shifted to low temperature, showing that the amount of weak acid sites increased and the amount of medium and strong acid sites decreased. Contrarily, on NH3 TPD profiles of VyT, the strong acid sites increased as loadings of vanadia increased. Above monolayer (V10T), a third peak appeared at the middle temperature range.
The TPR of V2O5/Al2O3 showed only one peak at lower temperature. It was attributed to the reduction of the monovanadate species. There are two reduction peaks in the TPR profiles of V2O5/TiO2 under monolayer coverage (V2T and V8T): they were attributed to the reduction of mono- and poly-vanadate species. When the coverage was over the monolayer (V10T), a third peak appeared at higher temperature, it might be the reduction of V2O5 crystallites.
Conclusions
- When the loadings of vanadia rose, the amount of weak acid sites on VxA increased whereas, the quantity of strong acid sites on VyT increased.
- V205 supported on Al2O3 was reduced more easily than on TiO2 because its theoretical monolayer coverage was lower.
References
[1] B. Grzybowska-Swierkosz, Appl. Catal. A, 157 (1997) 263- 310
[2] M.A. Reiche, M. Maciejewski, A. Baiker, Catal. Today, 56 (2000) 347-355
[3] J.W. Niemantsverdriet, Spectroscopy in Catalysis, Wiley-VCH, 2000
[4] G. C. Bond, Appl. Catal. A, 157 (1997) 91-103.