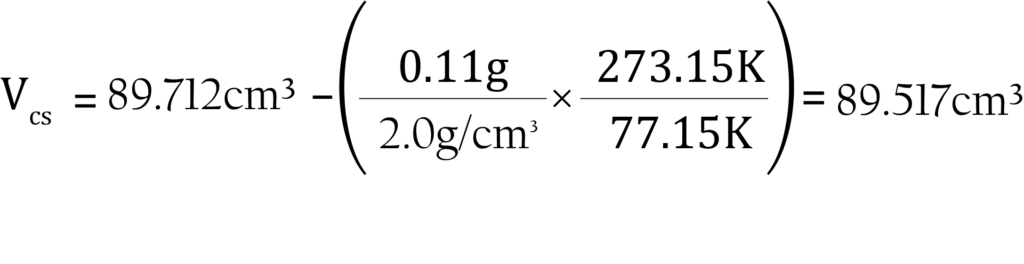Many microporous materials, such as zeolites and activated carbons, trap and hold helium in their complex pore structures for many hours after being exposed to helium. Helium trapped in micropores can interfere with the analysis at low pressures, causing an “S-shaped” curve at the lower end of the isotherm*. For this reason, it is recommended that you enter the warm and cold free-space volumes when performing micropore analyses, thereby avoiding exposure of the sample to helium. Two techniques can be used for determining warm and cold free-space values.
The first method is to perform a short analysis on the sample after partial degassing (one pressure point with no incremental dosing), but prior to final sample preparation. Measure the free space during this analysis. The measured free-space values will be printed on the report and may then be entered into the sample file after more thorough sample preparation.
The second, more precise method requires prior testing with empty sample tubes (blank tube analysis) that will be used later for the analyses. The measured free-space data can be used thereafter on every analysis performed using these sample tubes. This small initial investment of time will save considerable time later. Perform an empty tube analysis on each sample tube you intend to use for micropore analysis.
Measure the free space of each sample tube, using the low pressure dosing option to obtain a full isotherm with the analysis gas. The following recommendations should be followed to improve the quality of your micropore analyses.
- Be consistent in using seal frits; for example, use the same seal frits for the analysis as you did for the empty tube test.
- The cold free space is dependent on the adsorptive and the bath temperature; perform a test for each bath temperature (for example; liquid nitrogen, liquid argon, or others) to be used.
- The top of the isothermal jacket must be in the same position for the sample analysis as it was for the empty tube test.
- Correct the free-space volumes obtained from the blank tube for the volume displaced by the sample.
* Refer to Application Note No. 105 for additional information on the effects of helium retention in micropores.
You will need the following to perform a blank tube analysis:
- A clean sample tube
- A seal frit
- An isothermal jacket
- Default sample mass of 1 gram
The blank tube analysis should be completed in approximately four hours. It is important to follow normal recommendations for filling the dewar as provided in the instrument operator’s manual. Do not overfill the dewar, this will result in unpredictable free-space errors.
- Fit a clean sample tube with a seal frit and an isothermal jacket; do not use a filler rod*. Then degas the sample tube on the analysis port for one hour at 250 °C.
- Create a sample file, specify the following parameters:
| Preparation | Fast evacuation Evacuation time: 0.1 hours. |
| Free space | Measure Lower dewar for evacuation Evacuation time of 0.1 hours |
| Po and T | Measure P0 at intervals during analysis. Calculate the analysis bath temperature from these values. Measurement interval: 120 minutes. |
| Dosing | Maximum volume increment 3 cm3/g Absolute pressure tolerance 5 mmHg Relative pressure tolerance 5% Low-pressure incremental dose 3 cm3/g Equilibration delay: Minimum 0 h, Maximum 999 h. |
| Equilibration | Equilibration interval: 10 seconds |
| Backfill | Backfill sample at start and end of analysis; nitrogen for backfill gas. |
| Pressure table | A single point of 0.95 p/po. |
* A filler rod will interfere with pressure measurements and limit the quality of the micropore data. At low pressures (below 10-3 relative pressure), thermal transpiration becomes a significant effect. Thermal transpiration causes the pressure at the sample (77 K) to be significantly lower than the pressure at the transducer (approximately 300 K); the filler rod prevents the true pressure from being properly calculated.
3. Perform a blank tube analysis using the sample file you created (step 2). Figure 1 is an example of a blank tube analysis.
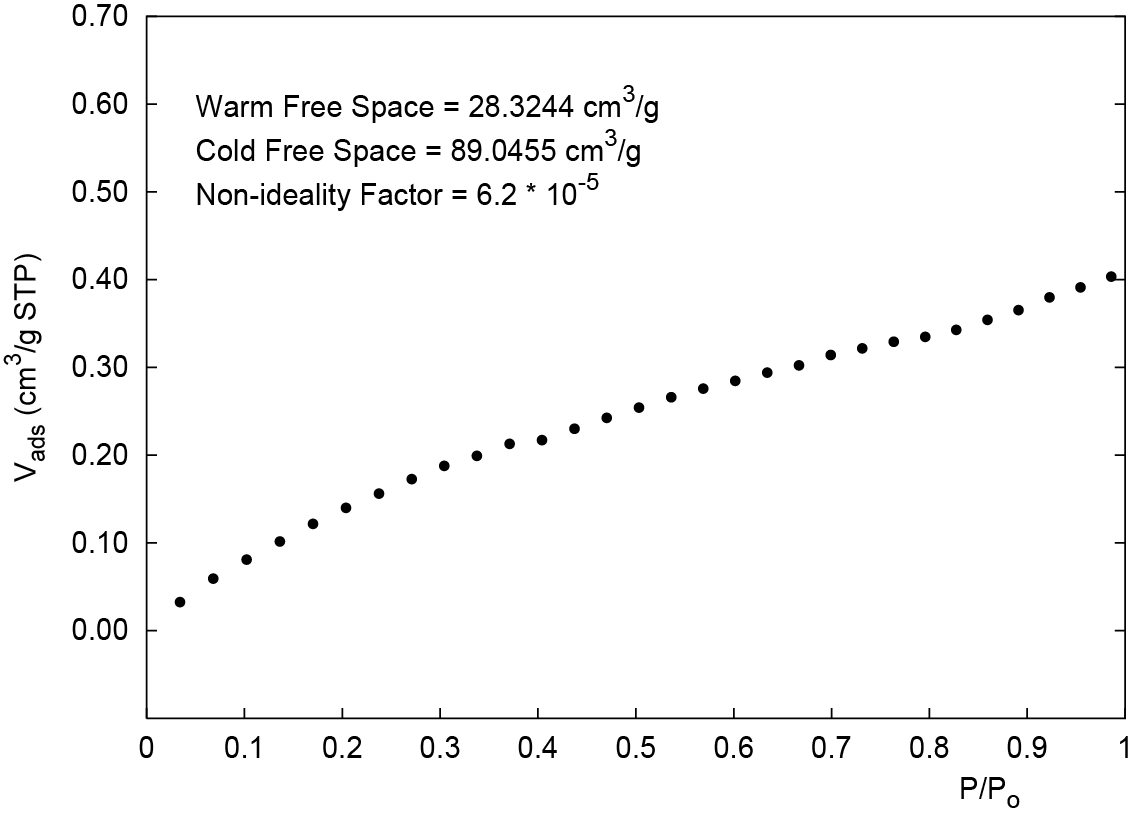
If you are using a cryogenic bath such as liquid nitrogen or liquid argon, it is important to optimize the adsorptive parameters and the free-space values (described in Step 4).
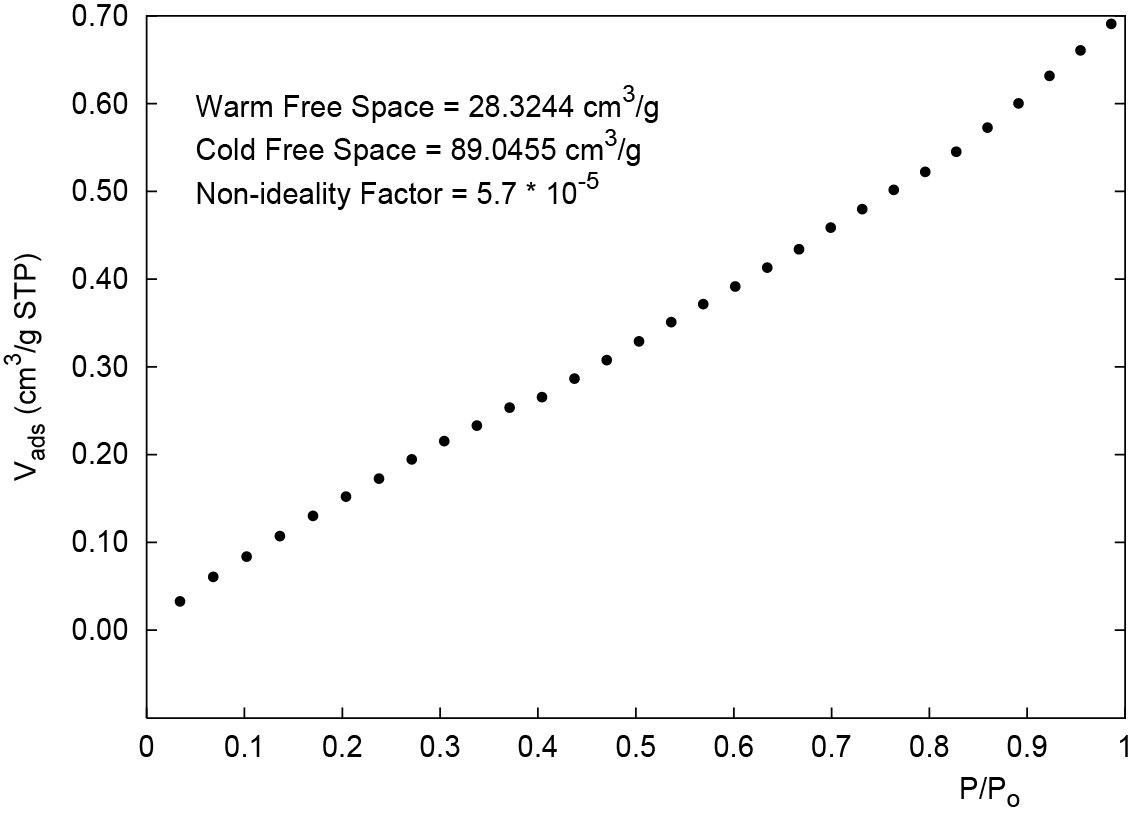
4. In the sample file for the blank tube analysis, click the Adsorptive Properties tab. In this example for nitrogen, reduce the non-ideality factor approximately 10% from 6.2 * 10-5 to 5.7 * 10-5. This will linearize the isotherm as shown in Figure 2. This correction is used to compensate for the portion of the empty tube that is not at liquid nitrogen temperature (77 K).
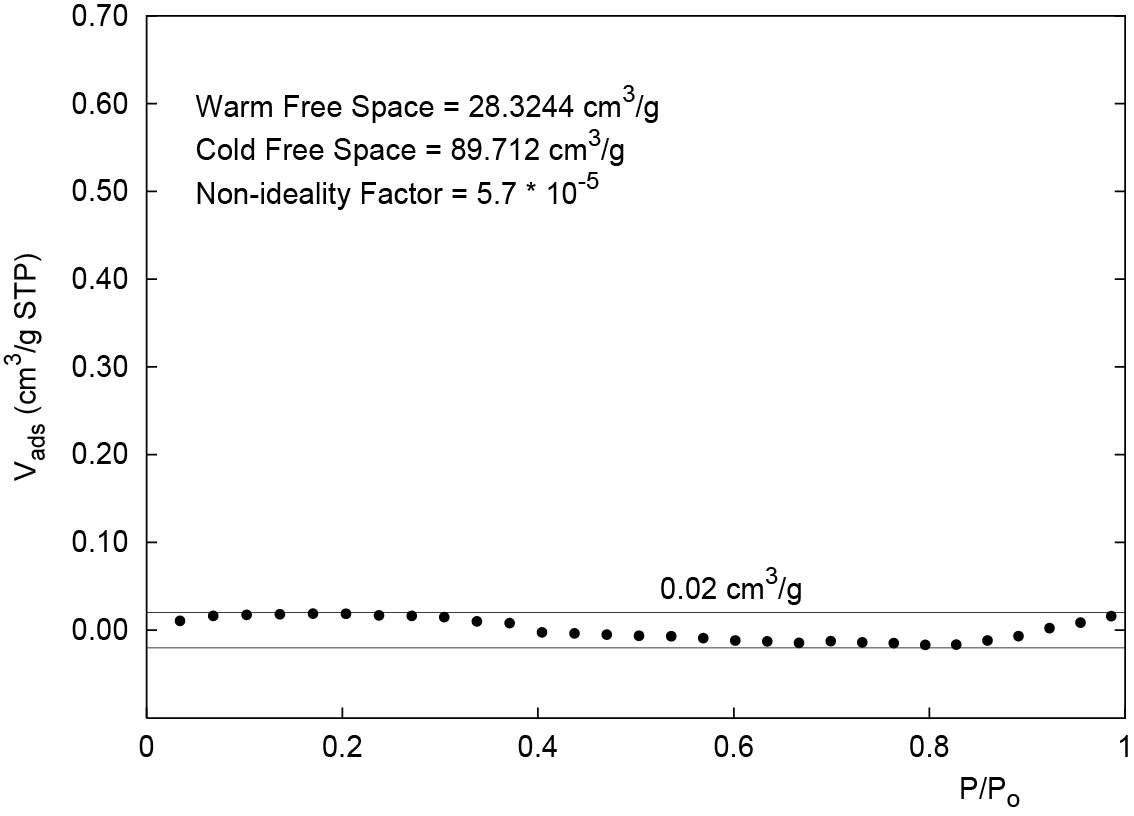
- To further minimize the blank error, add the volume at 0.95 P/P0 (Figure 2) to the cold free space. In this example (Figure 3), the cold free space is increased from the measured value of 89.0455 cm3/g to the optimized value of 89.712 cm3/g. The baseline error is reduced to less than ± 0.02 cm3/g.
6. To make the correction of the sample volume, subtract the amount of gas displaced by the sample. The calculations are simple and are given here with a brief explanation of their derivation. Remember to use the appropriate free-space values for each bath temperature used, and state all temperatures in Kelvin.
a. To calculate the warm free space:
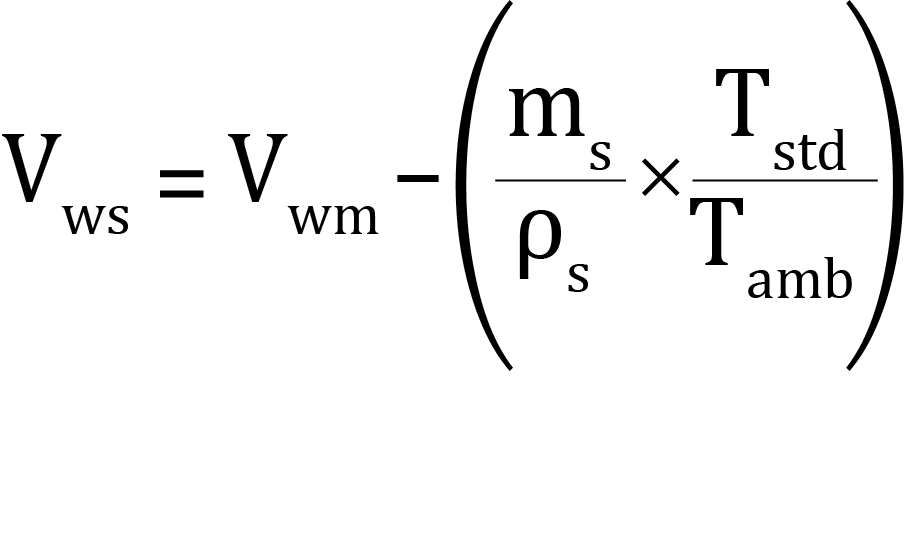
where:
Vws = calculated warm free space with sample present (standard cm3)
Vwm = warm free space measured for the empty tube (standard cm3)
ms = mass of sample to be analyzed (grams)
ρs = approximate sample true density (grams/cm3)
Tamb = ambient temperature (Kelvin)
Tstd = standard temperature (273.15 Kelvin)
b. To calculate the cold free space:
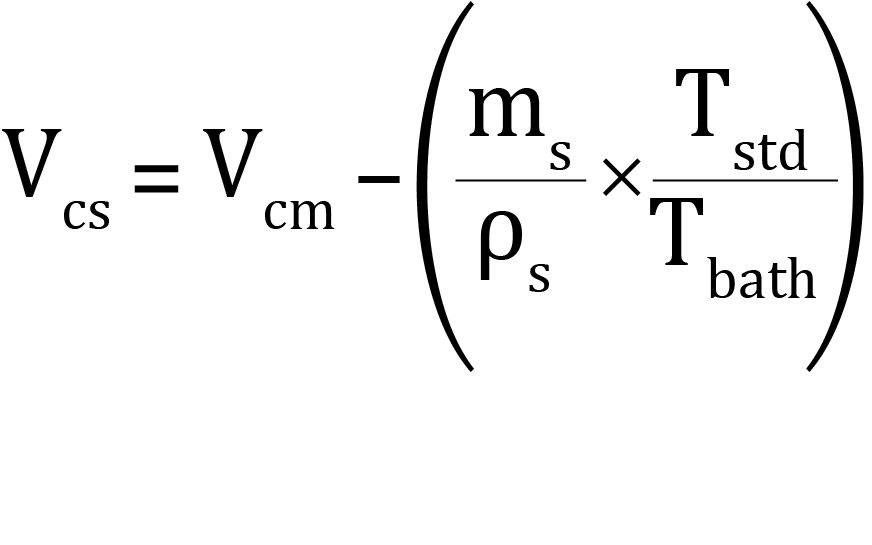
where:
Vcs = calculated cold free space with sample present (standard cm3)
Vcm = cold free space measured for the empty tube (standard cm3)
ms = mass of sample to be analyzed (grams)
ρs = approximate sample true density (grams/cm3)
Tbath = bath temperature (Kelvin)
Tstd = standard temperature (273.15 K)
- In the sample file for your next analysis, select Enter Free Space and enter the calculated values.
Example of Free-Space Calculations
The sample is 0.11 grams of activated carbon with a density of 2.0000 g/cm3. The room temperature is 22 °C or 295.15 K. The analysis tube has been measured previously in a nitrogen bath. The measured warm and cold free spaces are 28.3244 and 89.712 cm3 STP atm-1, respectively. The analysis will be performed with nitrogen at liquid nitrogen temperature, 77.15 K.
The warm free space is calculated as:
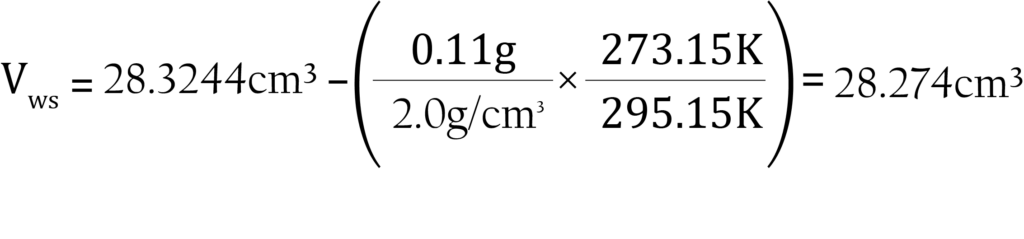
The cold free space is calculated as: