Physical characterization of pharmaceutical excipients is not only a requirement but can also provide data that can be predictive in nature regarding the performance of final dosage forms including tablets, capsules, inhaled dosage forms, transdermals, and others. Manufacturers generally provide some of this physical testing data, such as particle size. In the case of particle size data, the manufacturer’s specification may be wider than is actually acceptable for a particular process or product. Additionally, other tests may not be reported such as surface area, density, or porosity. In some cases, this data may provide insight into how a particular material will behave in a given process (flow, blending, compression) or final dosage form (disintegration, dissolution, bioavailability).
With implementation of Quality by Design, design space, risk analysis, and control strategies as outlined in ICH Q8, Q9, and Q10, increasing the knowledge base around excipients and APIs can aid in a company’s ultimate understanding of their materials and what effect they may have in a formulation.
Lactose and microcrystalline cellulose are two of the most common excipients used in solid oral dosage forms.
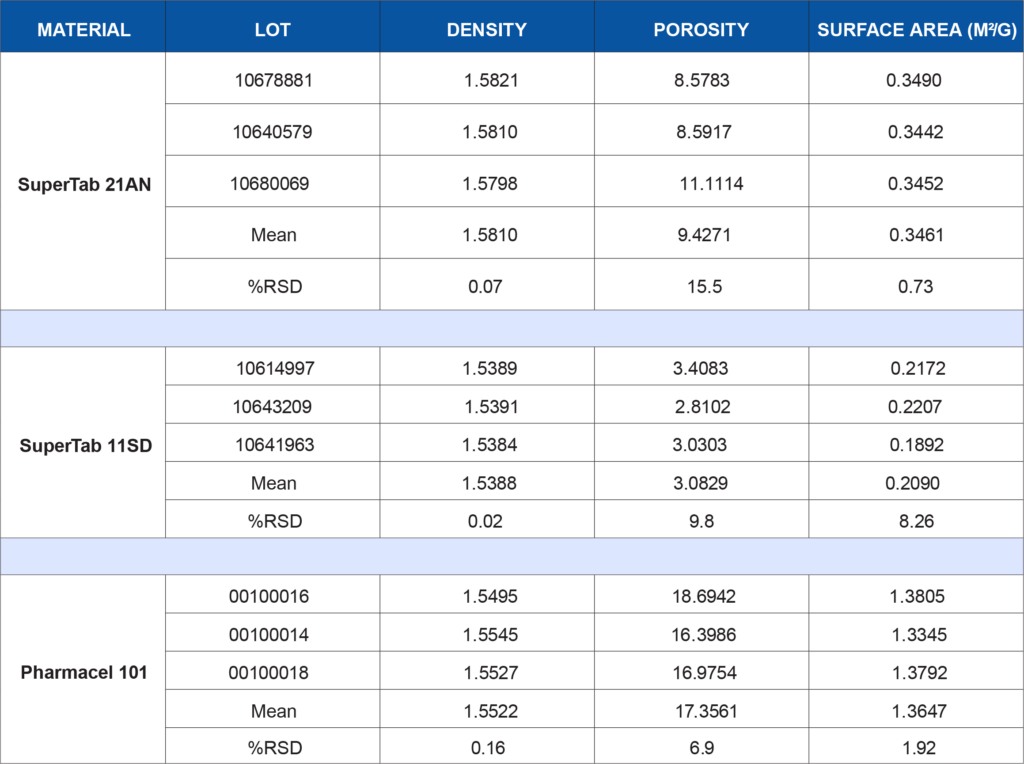
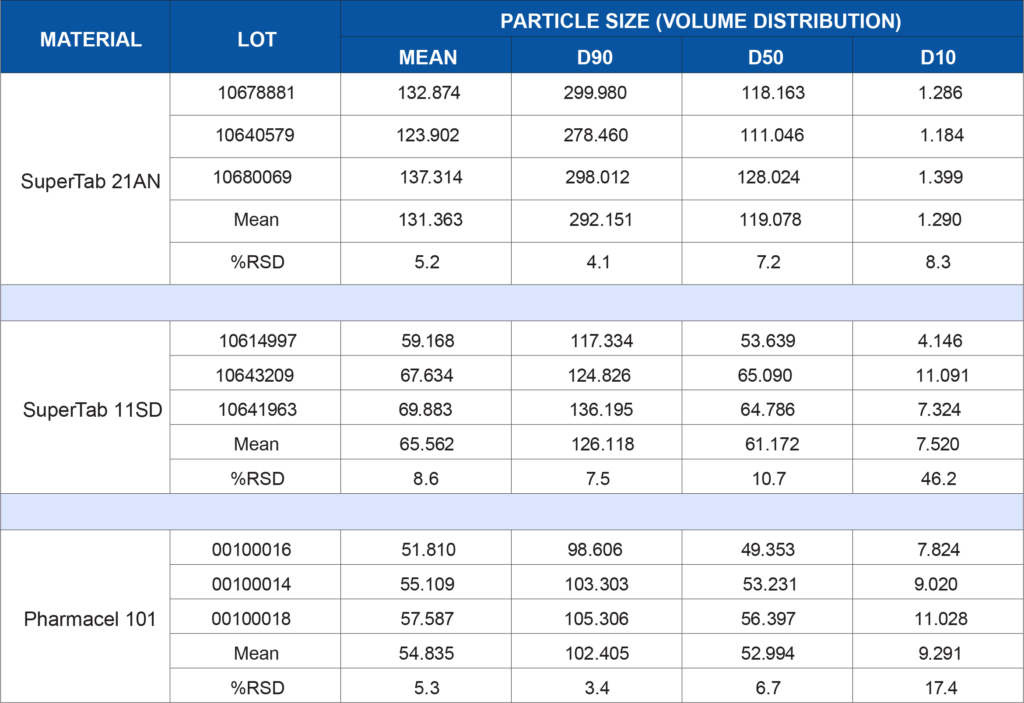
Lot-to-lot or supplier-to-supplier variation in either of these materials, particularly when they comprise the bulk of a formulation, could lead to unwanted issues. In this example, both of these excipients have been subjected to a battery of tests to demonstrate the degree of consistency of the materials. Three lots of each material were tested to simulate a raw material vendor qualification study. Anhydrous lactose (SuperTab 21AN), spray-dried lactose (SuperTab 11SD), and microcrystalline cellulose (Pharmacel 101) were provided by DFE Pharma. Each material was tested for the following characteristics:
- True or skeletal density by helium pycnometry on the AccuPyc 1340
- Porosity by mercury intrusion porosimetry on the AutoPore IV 9500
- BET specific surface area using krypton gas on the ASAP 2420 Surface Area Analyzer
- Particle size distribution by laser light scattering on the Saturn DigiSizer II
The following table summarizes the data generated for each test listed above. Depending on a company’s application or internally developed specification, the data may be utilized to show lot-to-lot similarity or may show that additional controls are needed to ensure the material is acceptable for use in a specific application. There is no generic wrong or right data set for every process or product. What the data means to you is application-dependent. If qualifying a new raw material supplier, does this data show equivalence?
Or is there a critical parameter that needs tighter control due to unwanted effects on a product performance characteristic such as dissolution rate? Clearly, the data generated for this study is more comprehensive than reliance on vendor specifications alone. This data in combination with corresponding product performance data can increase the assurance level that the product being manufactured will be more consistent, robust, and perform as intended once administered to the patient.
Conclusions
Monitoring raw materials is an important part of the overall control strategy in pharmaceutical formulations. Knowledge of physical characteristics can be beneficial when developing a design space or control strategies to ensure quality is built into processes and products and, in combination with final product performance, can help to identify critical process parameters and critical quality attributes of starting materials and final dosage forms.
The additional testing demonstrated in this study have shown that a more thorough examination of materials may help ensure consistency of material from a particular supplier, provides a battery of testing that can be performed to qualify new material suppliers, and may generate data that could be predictive in nature regarding process and/ or product performance. By building this knowledge base around your starting materials, many instances of undesirable process or product performance that may occur will result in troubleshooting with more “tools” in your “toolbox”.