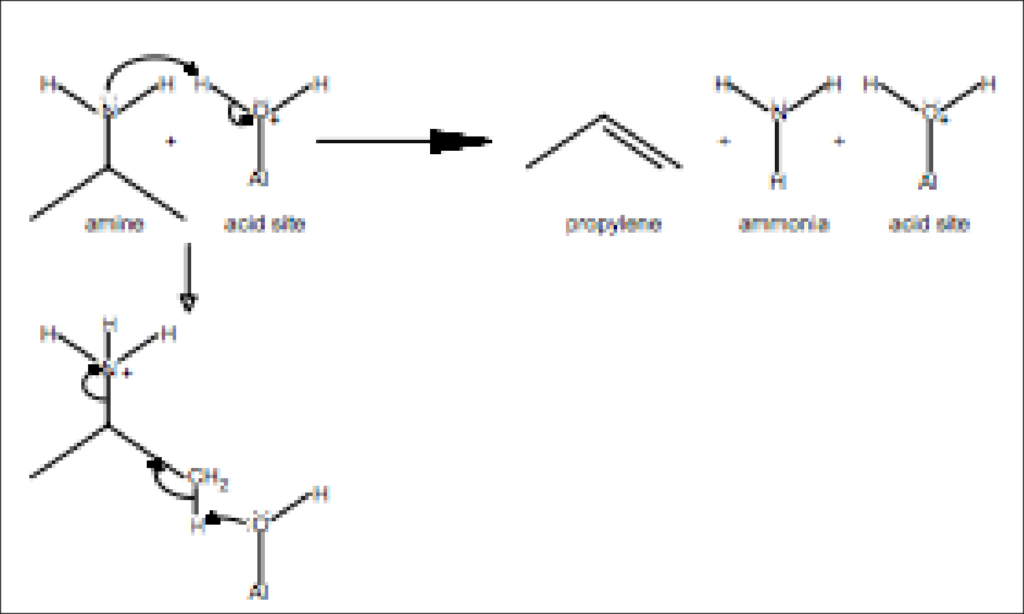
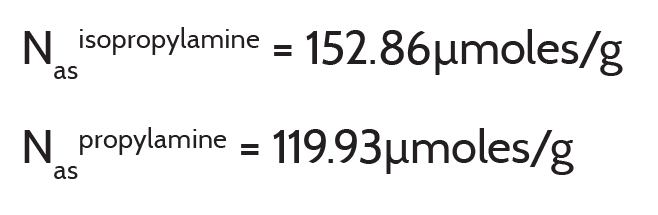The Brønstead acidity of zeolites and other catalysts is of keen interest, as this affects the kinetics of reactions. Characterization of these sites is consequently very important and is often performed according to the ASTM D 4824 ammonia chemisorption method. An alternative, but comparable, characterization method involves pulse chemisorption of propylamines followed by a temperature-programmed desorption (TPD) combined with a mass spectrometry analysis of propylene (see Figure 1). A complete characterization analysis can be performed using the AutoChem analyzer with the mass spectrometer and vapor generator accessories.
Materials
The zeolite used in this application contained hydrogen cations and had a silica-to-alumina ratio of 280:1. Isopropylamine (>99.5% GC) and propylamine (>99.0%GC) were used, separately, as reagents. Propylene (>99%) was also used for calibration purposes.
Preparation
A ZSM-5 sample can contain various cations, including ammonium and hydrogen. Cations in these compounds can be converted into hydrogen cations by means of a temperature ramp. The sample was activated first by heating to 500 °C at 10 °C/min in an inert helium environment, then cooling to 200 °C, the analysis temperature.
Analysis
The activation of the ZSM-5 was followed by pulse chemisorption. During this step, ten injections of propylamine vapor were dosed onto the sample (to ensure the sample was saturated) by means of an inert gas, helium, flowing through a 5-cm3 loop. The last part of the analysis involved a temperature-programmed desorption (TPD). At this step in the analysis, the mass spectrometer began scanning for propylene, the product of interest. Data were collected during a temperature ramp from 200 °C to 500 °C.
Data
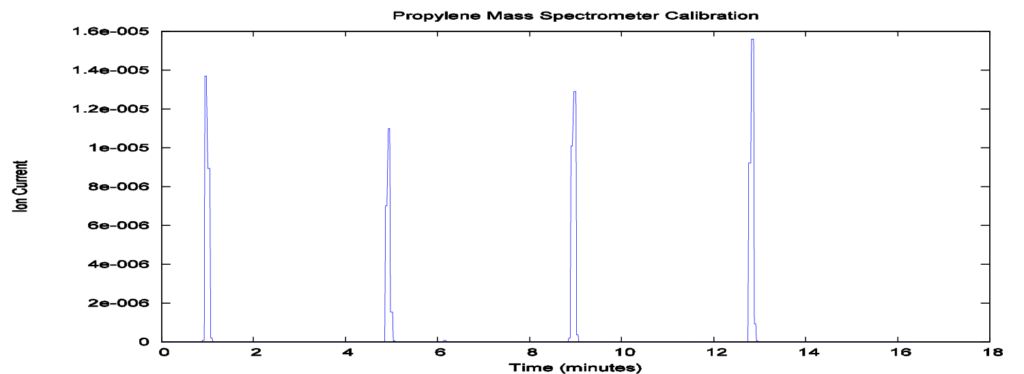
To obtain quantitative data, the mass spectrometer must be calibrated by injecting a known volume of the gas to be detected (propylene), Vcal, through the septum with a highprecision syringe. The peak area of the mass spectrometer signal can be obtained using the AutoChem peak-editing software. To increase the accuracy of the calibration, injections may be made until peaks are similar in area. The areas can then be averaged to give a general conversion factor between mass spectrometer peak area and actual gas volume, allowing for the acidity of zeolites to be calculated. Figure 2 gives an example of this procedure.
Additionally, Figures 3 and 4 show that a thermal conductivity detection method includes residual amine and ammonia from the chemisorption, whereas mass spectrometer detection isolates the propylene signal, allowing the concentration of acid sites to be calculated.
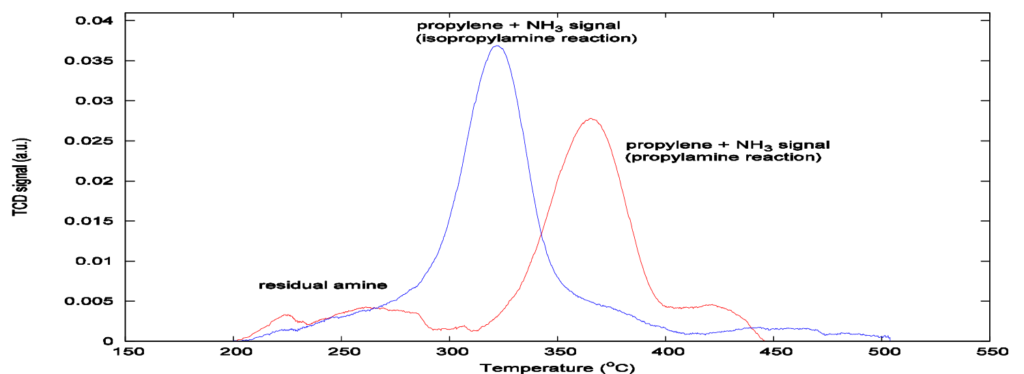
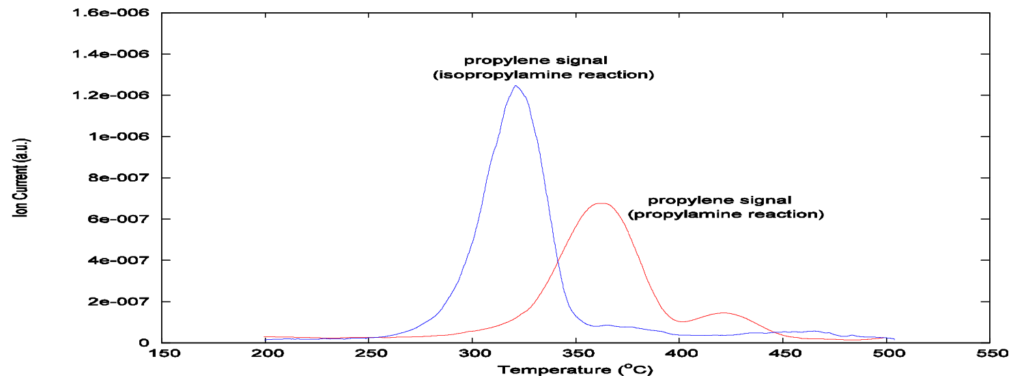
After obtaining the peak area by integration of the propylene signal from Figure 4, Apms, the acid site concentration, Nas, can be calculated as follows:
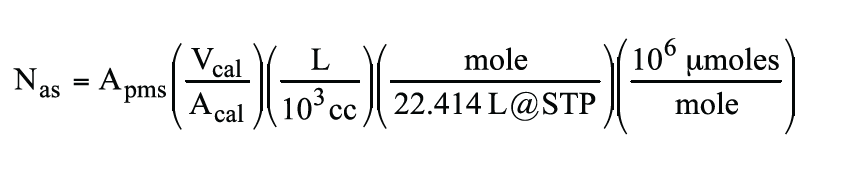
Below are calculated values that correspond to the data in Figure 4. The concentration values are expressed in units of micromoles of acid sites per gram of zeolite characterized.