Introduction
Zeolites are extensively used in many applications as ion exchange agents, adsorbents, and catalysts. Characterizing the acidity of a zeolite is important to design and optimize it for a catalytic activity of interest. Among many methods developed for characterizing the acid sites of a zeolite, the Temperature Programmed Desorption technique is one of the most widely used in the industry. It provides quick and reproducible means to characterize the number of acid sites present, relative acidity, and heat of desorption of each site when ammonia is employed as the basic probe. Furthermore, the Bronsted acid site concentration can be characterized using an alkyl amine as the probe, which undergoes Hofmann elimination only by the Bronsted acid sites present on the surface of a zeolite.
Temperature Programmed Desorption
Temperature Programmed Desorption (TPD) requires multiple thermal treatments. The
experimental procedure includes preparation of the sample, sorption of the probe, and finally
the TPD as shown in Figure 1.
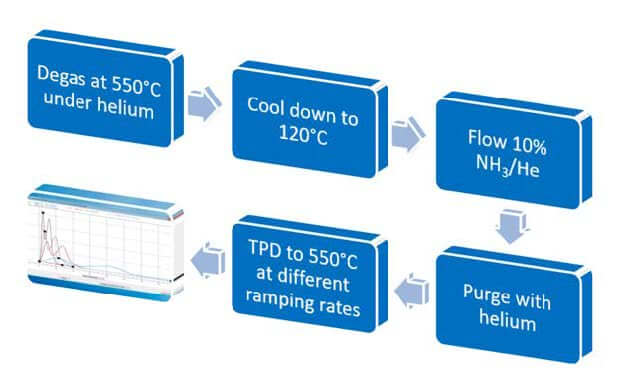
The multiple exposure to heat can pose a disadvantage to calculate the heat of desorption of a zeolite accurately when it is susceptible to degradation or structural changes due to thermal treatments. When analyzing such zeolite susceptible to changes under thermal treatments in this manner, a decrease in the amount of ammonia desorbed is often observed as the experiment progresses.
Beta Zeolite
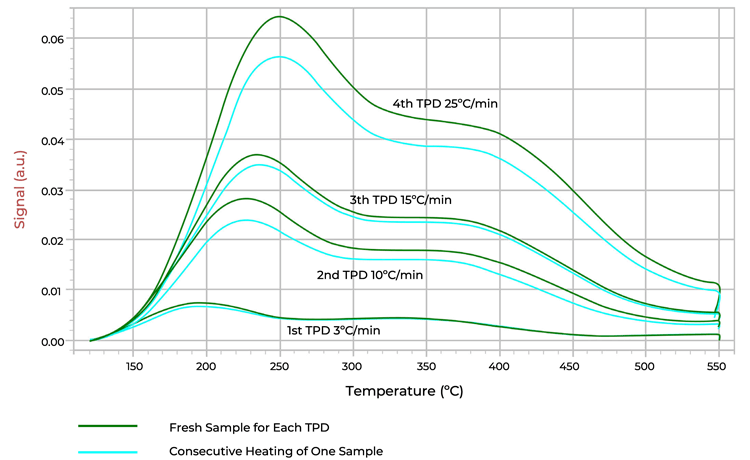
Beta zeolite is known to have amorphous content in its structure with defective sites that causes instability when thermally treated. Figure 2 shows how beta zeolite loses its acid sites as it is heated multiple times. By the time it is heated up for the fourth TPD experiment, a significant difference in the amount of ammonia desorbed is observed between the fresh sample and the sample that was heated multiple times.
ZSM-5
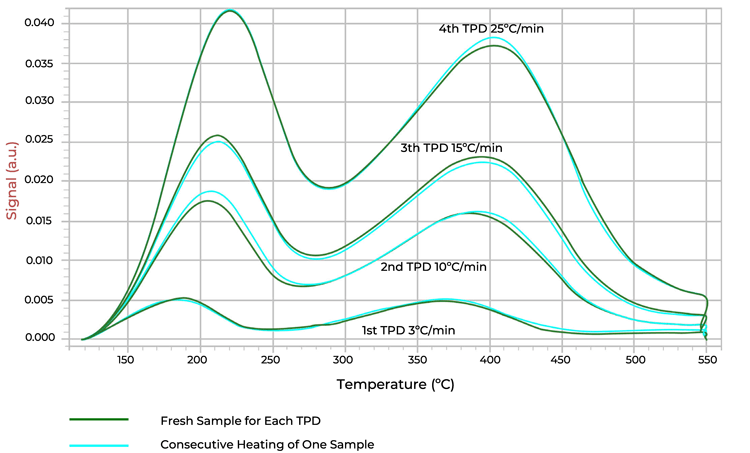
In comparison, the differences are negligible for ZSM-5, which is known to have more stability in heat due to is crystalline structure as shown in Figure 3.
Heat of Desorption
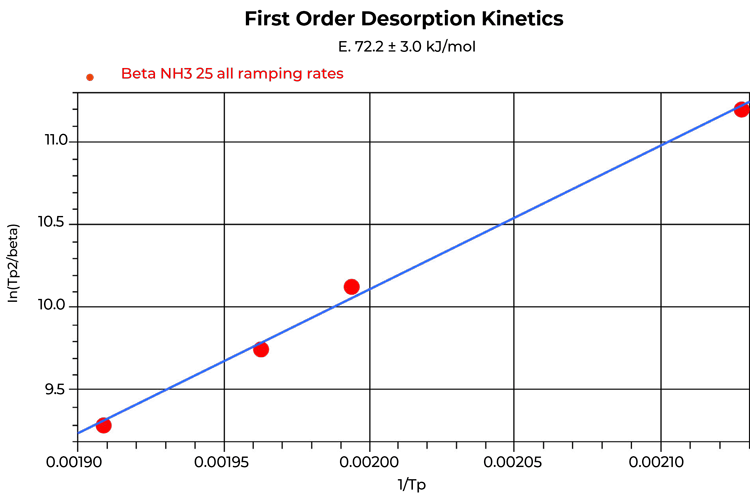
The heat of desorption is the activation energy required for the ammonia molecule to desorb from an acid site, so it relates to the binding strength of the site. It is calculated by using a first order kinetic model applied to several TPD experiments of multiple ramping rates, commonly performed consecutively with different ramping rates on the same sample. The ramp rates used for the experiments were chosen carefully since the first order kinetics should be applied over more than one order of magnitude to have the points reasonably distributed on the plot. The heat of desorption calculated from the consecutive TPD experiment completed on beta zeolite was 72.2 KJ/mol, whereas the separate TPD experiments using the fresh sample for each TPD resulted in 80.1 KJ/mol as shown in Figures 4 and 5.
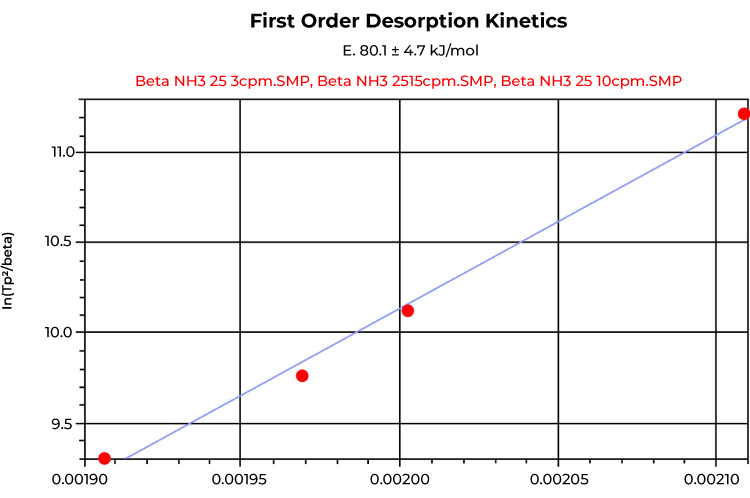
Conclusion
Ammonia TPD is a quick and easy measurement to determine the number of acid sites and the quantity of ammonia desorbed gives insight to the total acidity of a zeolite. The effect of structural changes with heat should be considered to determine the experimental approach for more accurate calculation for the heat of desorption. A fresh sample should be prepared for each TPD especially if the sample is known to degrade at high temperatures or if stability information is unknown. The AutoChem III MicroActive software is able to plot the first order kinetics from several different sample files to enable users to choose either consecutive or separate TPD experiments depending on the nature of the sample.