Author: Paul A. Webb
This article is a condensed version of a more comprehensive article titled “Chemical Adsorption,” as an Analytical Technique.
Introduction
Catalysts are used in a variety of applications from the production of consumer goods to the pro-tection of the environment. Optimum design and efficient utilization of catalysts requires a thor-ough understanding of the surface structure and surface chemistry of the active material. Chemical adsorption (“chemisorption”) analysis techniques provide much of the information nec-essary to evaluate catalyst materials in the design and production phases, as well as after a peri-od of use. Although a catalyst and the reactants and products can be of many forms, this article addresses commonly used heterogenous catalysts.
Differentiating Physical and Chemical Adsorption
A distinctive characteristic of a solid material is a distribution of weak surface energy sites. Gas or vapor molecules can become bound to these sites. This generally describes the adsorption phenomenon. The quantity of molecules taken up by the surface depends on several conditions and surface features including temperature, pressure, surface energy distribution, and the surface area of the solid. A plot of the quantity of molecules adsorbed versus pressure at constant temperature is called the adsorption isotherm.
Physical adsorption (“physisorption”) is the result of relatively weak Van der Waal’s interaction forces between the solid surface and the adsorbate – a physical attraction. Physical adsorption is easily reversed.
Depending on the gas and solid, the adsorption phenomenon also can result in the sharing of electrons between the adsorbate and the solid surface – a chemical bond. This is chemical adsorption and unlike physisorption, chemisorption is difficult to reverse. A significant quantity of energy usually is required to remove chemically adsorbed molecules.
Physical adsorption takes place on all surfaces provided that temperature and pressure conditions are favorable. Chemisorption, however, occurs only between certain adsorbents and adsorptive species and only if the surface is cleaned of previously adsorbed molecules.
Under proper conditions, physical adsorption can result in adsorbed molecules forming multiple layers. Chemisorption, on the other hand, only proceeds as long as the adsorptive can make direct contact with the surface; it is usually considered to be a single-layer process.
A characteristic of physical adsorption is that almost all the adsorbed molecules can be removed by evacuation at the same temperature at which adsorption occurred. Heating accelerates desorption because it makes readily available to the adsorbed molecules the energy necessary to escape the adsorption site.
A chemically adsorbed molecule is strongly bound to the surface and cannot escape without the influx of a relatively large quantity of energy compared to that necessary to liberate a physically bound molecule. This energy is provided by heat and often very high temperatures are required to clean a surface of chemically adsorbed molecules.
Physisorption tends to occur only at temperatures near or below the boiling point of the adsorptive at the prevailing pressure. This is not the case with chemisorption. Chemisorption usually can take place at temperatures well above the boiling point of the adsorptive.
The Relationship of Chemisorption to Catalysis
A catalyst is a material that affects the rate of a chemical reaction. A catalyst cannot cause a reaction that otherwise would not occur; it only can increase the rate at which the reaction approaches equilibrium. The surface of an ‘active’ metal is composed of chemisorption sites. Supported catalysts are those on which finely divided grains of the active metal are deported on a support material. Those grains located on the surface of the support are available to react with the adsorptive. If the accelerated rate of reaction was simply due to an increased concentration of molecules at the surface, catalysis could result from physical adsorption of the reactants. This is not the case; chemisorption is an essential step, apparently altering the reactant (the adsorbed molecule) to make it more receptive to chemical reaction. The dependence of catalysis on forming active surface bond intermediates is one reason why chemisorption as an analytical technique is so fundamental in the study of catalysis.
Stages of a heterogeneous catalytic reaction cycle are:
1) diffusion (transport) of reactants to the surface of the catalyst
2) chemisorption of reactant(s)
3) surface reactions among chemisorbed species
4) liberation of products from catalysts
5) diffusion of products away from the surface of the catalyst to allow recycling to step 1
Predicting the efficiency of steps 1 and 5 is aided by analytical techniques such as physical adsorption and mercury porosimetry, which characterize the porosity of the catalyst bed, catalyst monolith, or the individual grains of catalyst material. Characterizing steps 2, 3, and 4 is the domain of chemisorption analyses.
Chemisorption Techniques & Methods for the Evaluation of Catalysts
Chemisorption analyses may be applied to determine a catalyst’s relative efficiency in promoting a particular reaction, or used to study catalyst poisoning and in monitoring the degradation of catalytic activity over time of use.
Isothermal chemisorption analyses are performed by two chemisorption techniques: a) static volumetric chemisorption, and b) dynamic (flowing gas) chemisorption. The volumetric technique is convenient for obtaining a high-resolution measurement of the chemisorption isotherm from very low pressure to atmospheric pressure at essentially any temperature from near ambient to 1000 ºC or greater.
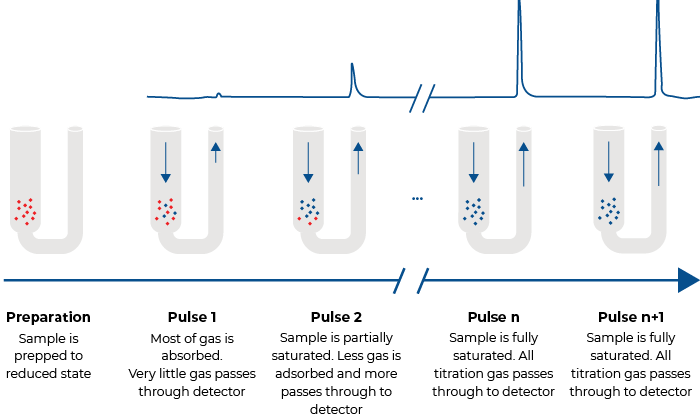
Pulse chemisorption, a flowing gas technique, typically is performed at ambient pressure. After the sample has been cleaned in a flow of inert gas, small quantities of a reactant are injected until the sample is saturated. A calibrated thermal conductivity detector (TCD) is used to determine the quantity of reactant molecules taken up by active sites upon each injection. Initial injections may be chemisorbed totally; upon saturation none of the later injections will be chemisorbed, indicating saturation. The number of molecules of gas chemisorbed is directly related to the active surface area of active material.
The quantity of gas chemisorbed per gram of sample combined with the knowledge of the stoichiometry of the reaction and the quantity of active metal mixed with support material during formulation of the catalyst allows the percent metal dispersion to be calculated. This can be an important indicator of the performance of the catalyst and an important economic measure of how efficiently the expensive active metal is being employed in a catalyst product.
Pulse Chemisorption
Temperature-Programmed Desorption (TPD), Temperature-Programmed Reduction (TPR) and Temperature-Programmed Oxidation (TPO) are three non-isothermal methods for characterizing catalysts. Temperature-programmed desorption typically does not employ a vacuum, better simulating conditions found in actual industrial applications. In the TPD analysis, materials are placed in a sample cell and pretreated to clean the active surfaces. Next, a selected gas or vapor is chemisorbed onto the active sites until saturation is achieved, after which the remaining molecules are flushed out with an inert gas.
Temperature (energy) is increased at a controlled rate while a constant flow of inert gas is maintained over the sample. The inert gas and any desorbed molecules are monitored by a thermal conductivity detector. The TCD signal is proportional to the quantity of molecules desorbed as thermal energy overcomes the binding energy. Quantities desorbed at specific temperatures provide information about the number, strength, and heterogeneity of the chemisorption sites
Temperature Programmed Desorption (TPD)
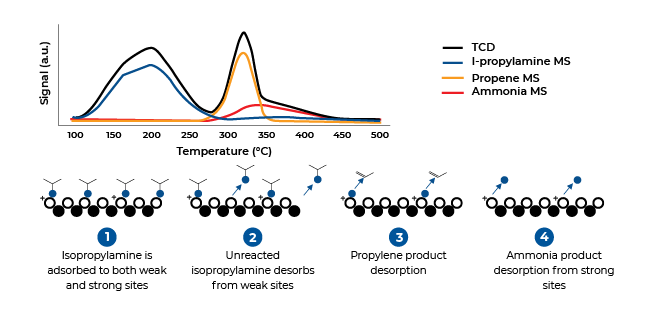
Temperature Programmed Reduction (TPR)
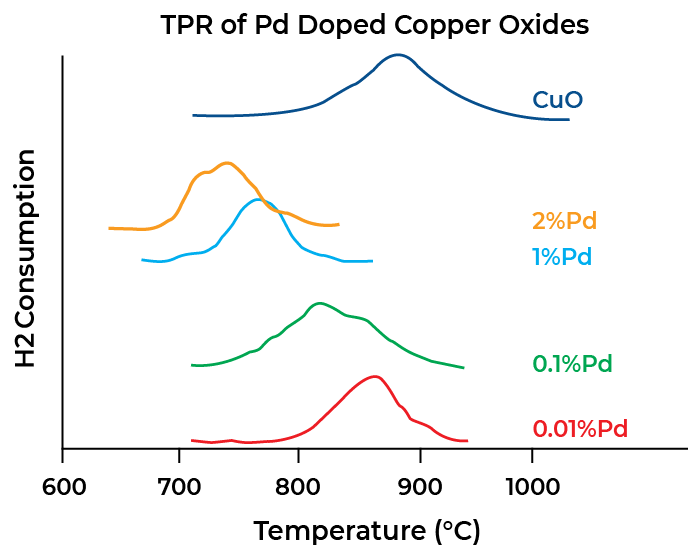
Temperature-programmed reduction is mainly used to study the reducibility of species such as metal oxides dispersed on a support. This involves flowing a stream of diluted hydrogen (or another reducing agent) over the sample as the sample temperature is increased. The quantity of hydrogen consumed and the temperature profile under which the reduction took place are measured. A plot of quantity of hydrogen consumed versus temperature can produce one or more peaks and the data obtained reveal the number of reducible species in the sample, as well as their activation energies.
Surface Energies
When a solid surface is exposed to an adsorptive, the most energetic sites are occupied first. The heat of adsorption at a specific degree of surface coverage (loading) can be calculated using the Clausius-Clapeyron equation. This expression describes the isosteric heat of adsorption in terms of pressure, temperature, and the gas constant and is particularly applicable to data obtained by volumetric adsorption techniques.
The isosteric heats of adsorption over a range of coverage can be obtained from adsorption isosteres, which are plots of pressure vs. temperature at a constant volume adsorbed. The isosteres are extracted from a family of isotherms obtained for the same material at different temperatures. The slope of an isostere plotted on a logarithmic scale (lnP vs 1/T)n provides one data point (qst, n), where n represents the degree of coverage associated with the isostere. A plot of similar points for different degrees of coverage describes the surface energy distribution as a function of coverage. This information aids in predicting the activity of a catalyst toward a specific chemical reaction at a specific temperature.
Activation energy also can be deduced from data obtained by the dynamic chemisorption tech-nique, particularly TPD. The process by this method is in the opposite direction as that described for static volumetric technique. In the present case, heat (energy) is applied and, as temperature increases, molecules are liberated in order of weakest bonding. The desorbed molecules are swept away and no readsorption is allowed to occur. The rate of change of surface coverage, or loading, is related to the rate of change in temperature.
The rate of simple molecular desorption may be modelled using 1st order kinetics commonly expressed as -kq, where k is the rate constant, the negative sign indicating a reduction in cover-age with time, and q represents the current degree of surface coverage.
The rate constant k can be expressed in Arrhenius form, A exp(-Ea/RT), where Ea is the activation energy for desorption, T is absolute temperature, and R the gas constant. A is known as the pre-exponential factor.
Combining the relationships and equations presented above ultimately yields an expression for activation energy in terms of variables that can be determined by TPD analyses.
Summary
Chemisorption is a fundamental process in heterogeneous catalysis. Understanding the chemisorption process associated with a catalyst and reactant is key to controlling the composition and manufacture of catalysts and for catalyst evaluation. Therefore, analytical instruments capable of measuring chemical and physical adsorption and desorption isotherms and those capable of analyzing temperature programmed reactions are powerful tools in the study of catalysis.