Introduction
The AccuPyc is widely used for analyzing both solids and powders. Little information is readily available for using a pycnometer to analyze liquids. The AccuPyc measures pressure of the system to determine volume and calculate density. Pressure generated by sample evaporation can diminish precision and system equilibrium might not be obtainable.
Vapor pressure is the equilibrium pressure on a liquid by its vapor within a closed system. The molecules of liquids are held together by physical bonds, that is Van der Waal forces. Liquids with weaker bonds create vapor more easily and have higher vapor pressure than liquids with stronger bonds and lower vapor pressure. An increase in temperature causes an increase in vapor pressure.
This document shows the results and conditions used to analyze various liquids with different vapor pressures on the AccuPyc and how selecting different parameters impact the analysis. Liquids that could not damage the instrument were used. Helium was used unless otherwise stated.
Analyzing Liquids
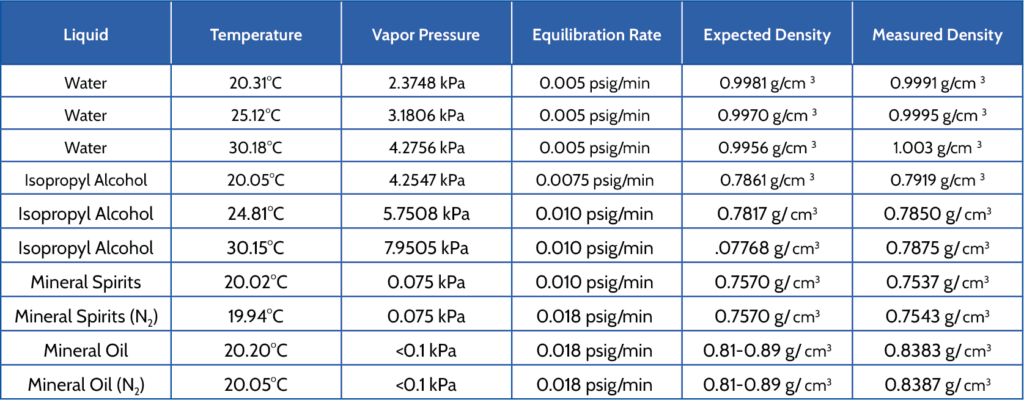
Water, isopropyl alcohol, mineral spirits, and mineral oil were analyzed using a 10cc TEC AccuPyc. Table 1 displays average temperature, vapor pressures, equilibration rates, expected density, and average measured density.
Liquids were placed in the AccuPyc for 10 minutes before beginning the analysis to ensure the liquid temperature would be uniform with the chamber. Equilibration rates were determined by selecting the default value of 0.005 psig/min and monitoring the equilibration rate during the analysis. If the monitored rate was not able to achieve the default value, a new obtainable rate was selected based on the monitored value.
Mineral spirits and mineral oil were analyzed with both helium and nitrogen. Results were consistent.
Equilibration Mode and AccuPyc Size Determination
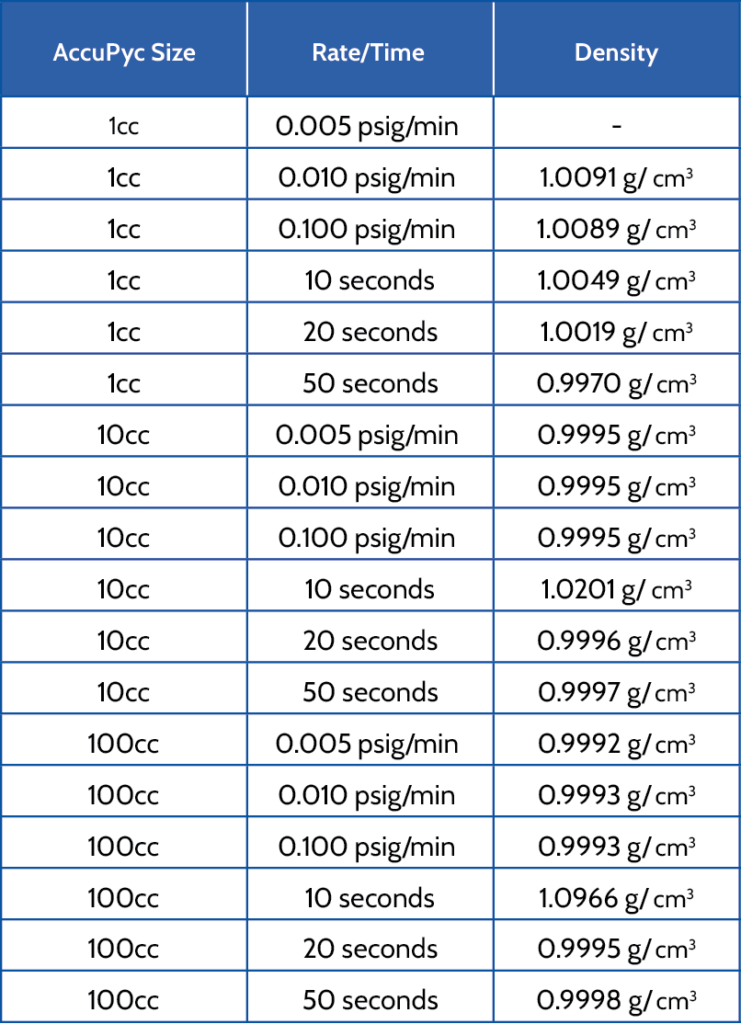
The AccuPyc has two different equilibration modes. Pressure can stabilize by an equilibration rate or a fixed time interval using the scan option.
Water was analyzed at room temperature under three different equilibration rates and three different time intervals for a 1cc, 10cc, and 100cc nominal volume AccuPyc. Five purges and five cycles were used for each analysis. The 1cc AccuPyc was not able to equilibrate using the 0.005 psig/ min equilibration rate and obtained the best data with using a higher time interval. Data obtained for the 10cc and 100cc AccuPyc’s with a low time interval was slightly higher compared to using a higher time interval or equilibration rates. Table 2 lists the results of each of the analyses.
Length of Analysis
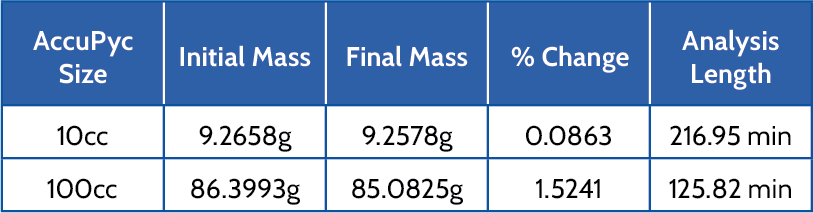
Longer analyses result in noticeable mass changes due to a slight amount of liquid being removed with each purge or cycle. Water was analyzed in a 10cc and 100cc AccuPyc at room temperature using 10 purges and 99 cycles. Table 3 displays the initial mass, final mass, percent change, and analysis length.
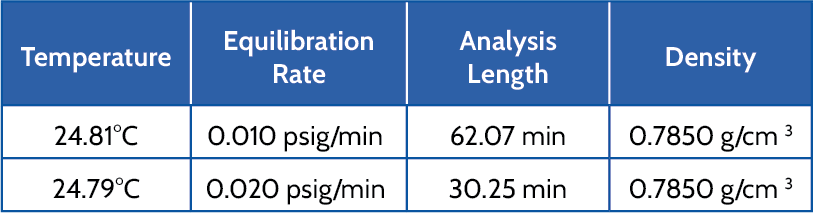
The equilibration rate also impacts the length of the analysis. Isopropyl alcohol was analyzed at room temperature, in a 10cc AccuPyc using 10 purges and 10 cycles, with two different equilibration rates. Table 4 displays the average temperature, equilibration rate, average measured density, and analysis length.
Conclusion
Analyzing liquids is similar to analyzing solids and powders on the AccuPyc. Attention should be paid to equilibration rates or time intervals, number of purges, and cycles. All measured densities are within range of expected densities based on the instrument’s accuracy.