Temperature-Programmed Desorption (TPD) is one of the most widely used and flexible techniques for characterizing the acid sites on oxide surfaces1-5. Determining the quantity and strength of the acid sites on alumina, amorphous silica-alumina, and zeolites is crucial to understanding and predicting the performance of a catalyst. For several significant commercial reactions (such as n-hexane cracking, xylene isomerization, propylene polymerization, methanol-to-olefins reaction, toluene disproportionation, and cumene cracking), all reaction rates increase linearly with Al content (acid sites) in H-ZSM-56-11. The activity depends on many factors, but the Brønsted-acid site density is usually one of the most crucial parameters.
There are three types of molecular probes commonly used for characterizing acid sites using TPD: ammonia, non-reactive vapors, and reactive vapors. TPD of ammonia is a widely used method for characterization of site densities in solid acids due to the simplicity of the technique. Ammonia often overestimates the quantity of acid sites. Its small molecular size allows ammonia to penetrate into all pores of the solid where larger molecules commonly found in cracking and hydrocracking reactions only have access to large micropores and mesopores. Also, ammonia is a very basic molecule which is capable of titrating weak acid sites which may not contribute to the activity of catalysts. The strongly polar adsorbed ammonia is also capable of adsorbing additional ammonia from the gas phase.
Larger non-reactive amines such as pyridine and t-butyl are often preferable alternatives to ammonia because their size permits access to the pore size range required for catalytic cracking reactions and they titrate only the strong and moderate acid sites. The most common application for these probes is the characterization of pyridine adsorption by infrared spectroscopy. However, the determination of extinction coefficients is difficult and IR of pyridine is typically used in a qualitative manner, rather than as a measurement of site densities.
The most commonly used reactive probes are the propyl amines. These amines are reactive and decompose to propylene and ammonia over Brønsted-acid sites. The temperature-programmed decomposition of amines is the most modern technique for measuring Brønsted-acid site concentrations. The method is based on the formation of alkylammonium ions (from adsorbed alkyl amines that are protonated by Brønsted sites) that decompose to ammonia and olefins in a well-defined temperature range via a reaction similar to the Hofmann-elimination reaction.
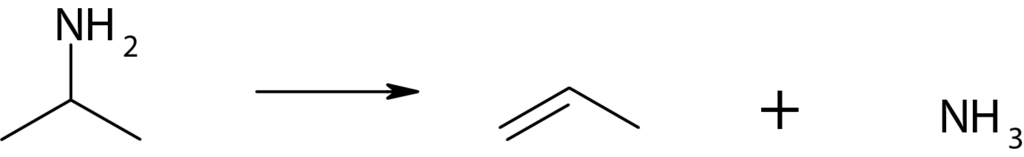
As long as the alkyl group can give up a hydrogen atom to form an olefin and the amine is small enough to access the Brønsted sites, the measured site densities are independent of the particular amine used to probe the sites. The technique is equally valuable for characterizing amorphous and crystalline solid acids.
The use of organic amines and other basic vapors is possible using Micromeritics’ AutoChem Series of instruments which provides internal heating of the lines, valves, and detector to prevent condensation of the experimental vapors.
Preparation
Samples are degassed at 100 °C for one hour in flowing helium to remove water vapor and to avoid pore damage from steaming which may alter the structure of zeolites. The samples are then temperature programmed to 500 ºC at a ramp rate of 10 °C/minute and held at that temperature for two hours to remove strongly bound species and activate the sample. Finally the sample is cooled to 120 °C in a stream of flowing helium.
Adsorption
Next the sample is saturated with the basic probe at 120 °C; this temperature is used to minimize physisorption of the ammonia or organic amines. For ammonia, two techniques are available to saturate the sample: pulsing the ammonia using the loop or continuously flowing ammonia. Pulsing the ammonia allows the user to compare the quantity of ammonia adsorbed (via pulse adsorption) to the quantity desorbed for the subsequent TPD.
Using the organic amines requires the use of a vapor generator; the sample must be saturated by using the built-in loop and pulse adsorption. The AutoChem vapor generator contains a temperature-controlled valve, reflux condenser, and flask for the probe liquid. Temperature-control allows precise control of vapor composition. Its use is imperative for liquids with high vapor pressures. The temperature zones for the AutoChem should be altered to reflect the use of vapors in the system; in particular, the temperature for the valves should be set to 110 °C. While using organic amines the vapor valve zone temperature should also be set to 110 °C to prevent condensation. The temperature of the condenser controls the liquid vapor pressure (and concentration of the vapor in the loop). An appropriate temperature can be obtained using the Antoinne equation to calculate the temperature required to obtain a vapor pressure or 0.1 to 0.2 bar which translates to 10 to 20% vapor composition by Dalton’s equation for partial pressures.
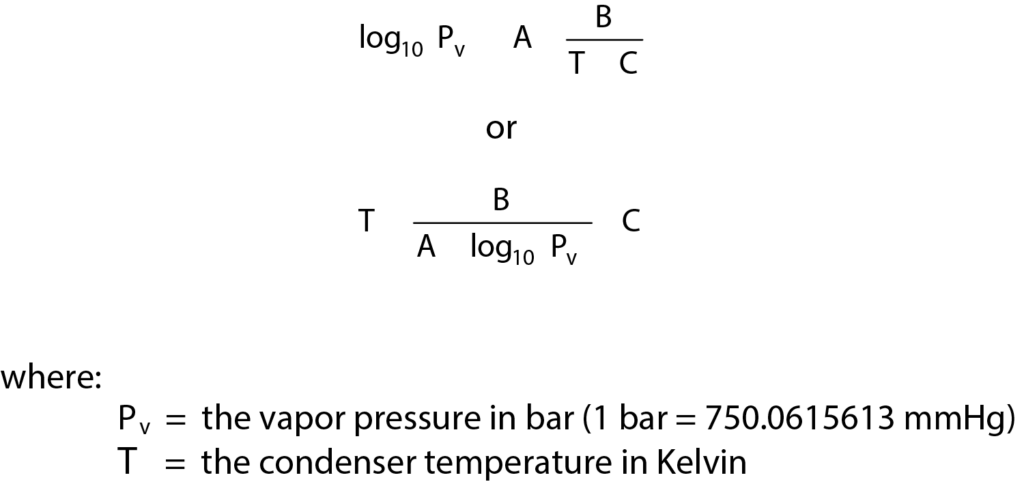
Antoinne constants are given in Table 1 for several common probes. The temperature of the liquid flask should be set to 5 – 10 °C above the temperature of the condenser but should remain below the boiling point of the liquid. The vapor generator is designed to provide constant vapor pressure and not boil the liquid.
After saturation with ammonia, pyridine, or propyl amine, the sample is purged for a minimum of one hour under a flow of helium to remove any of the physisorbed probe.
Desorption
The temperature-programmed desorption is easily performed by ramping the sample temperature at 10 °C/minute to 500 °C. It is a good rule of thumb that the end temperature during the TPD not exceed the maximum temperature used in the preparation of the sample. Exceeding the maximum preparation temperature may liberate additional species from the solid unrelated to the probe molecule and cause spurious results.
During the TPD of ammonia or the non-reactive probes (pyridine or t-butyl amine), the built-in thermal conductivity detector (TCD) will monitor the concentration of the desorbed species. For the reactive probes (propyl amines), a mass spectrometer is required to quantify the density of acid sites. For these probes, several species may be desorbing simultaneously: amine, propylene, and ammonia.
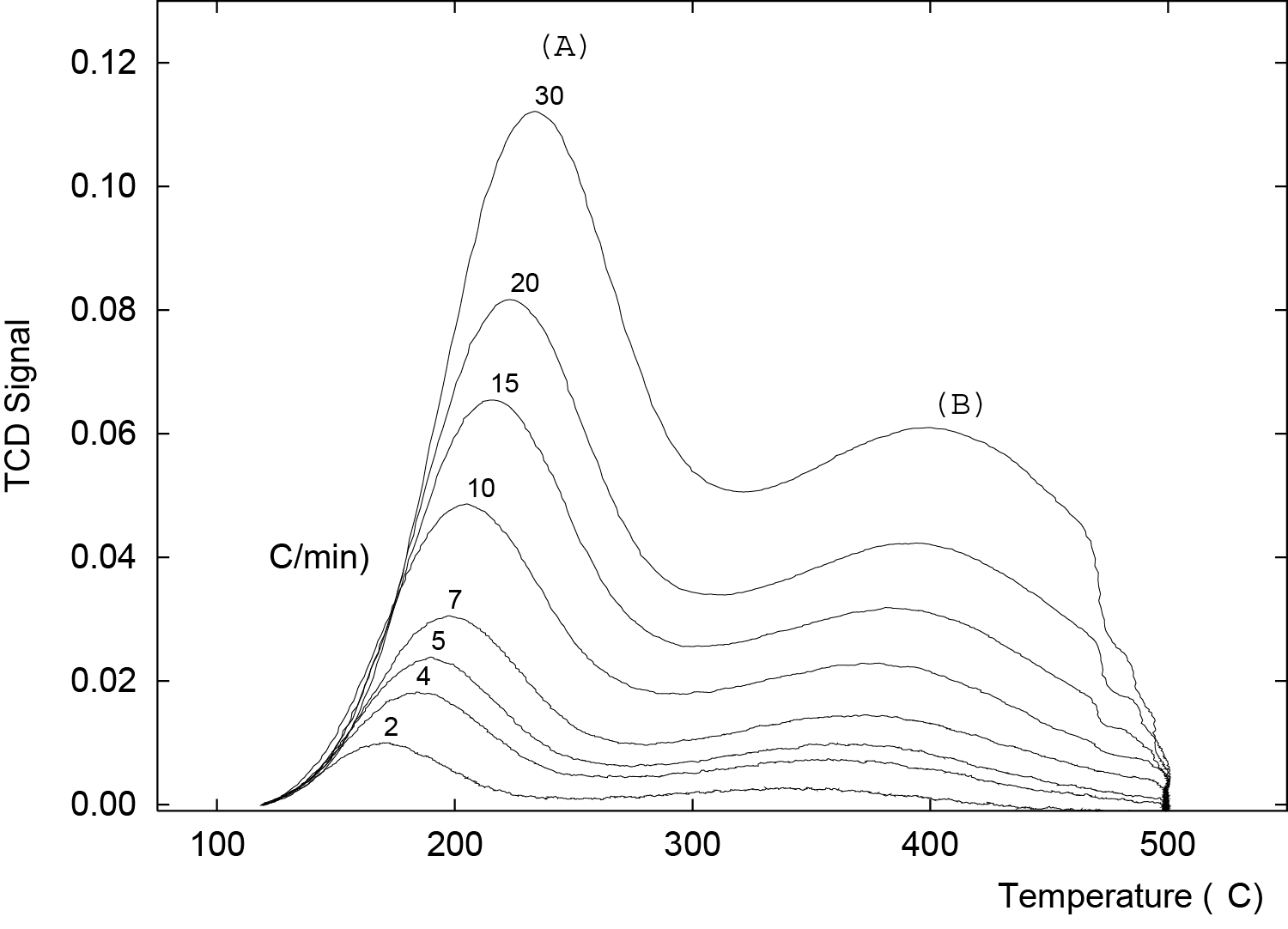
TPD profiles for ammonia desorption are obtained by raising the sample temperature according to a specific heating rate. Eight TPD profiles, obtained at heating rates of 2, 4, 5, 7, 10, 15, 20, and 30 °C/min are shown in Figure 1. This zeolite clearly shows two distinct acid sites A and B.
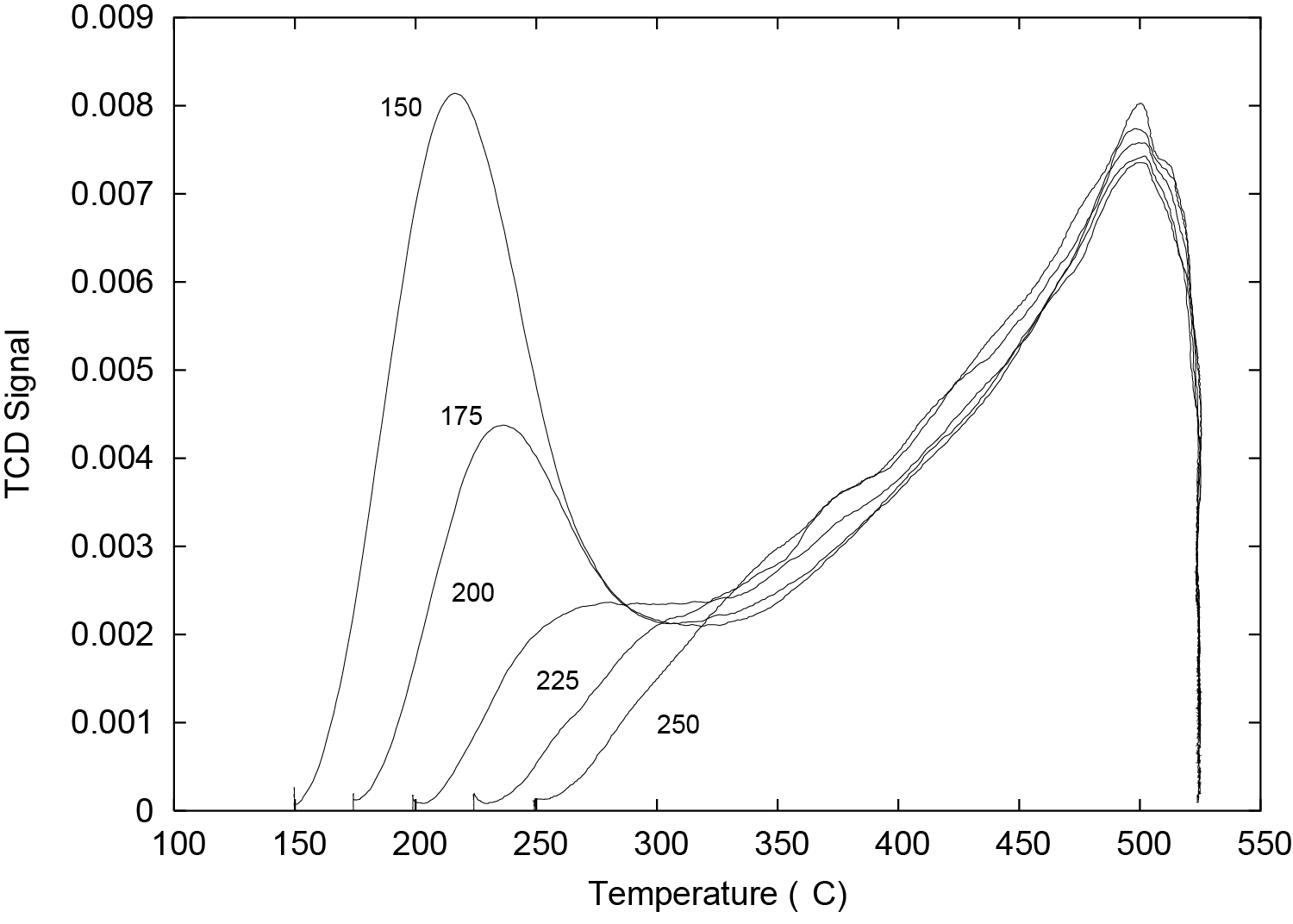
TPD profiles for pyridine desorption were obtained by using several different adsorption temperatures (150, 175, 200, 225, and 250 °C) and then raising the sample temperature at 10 °C/min; these TPD profiles are shown in Figure 2. These TPD spectra of pyridine demonstrate the effect of temperature upon the quantity of weakly sorbed pyridine and also show that the quantity of strongly sorbed pyridine does not change with repeated cycling.
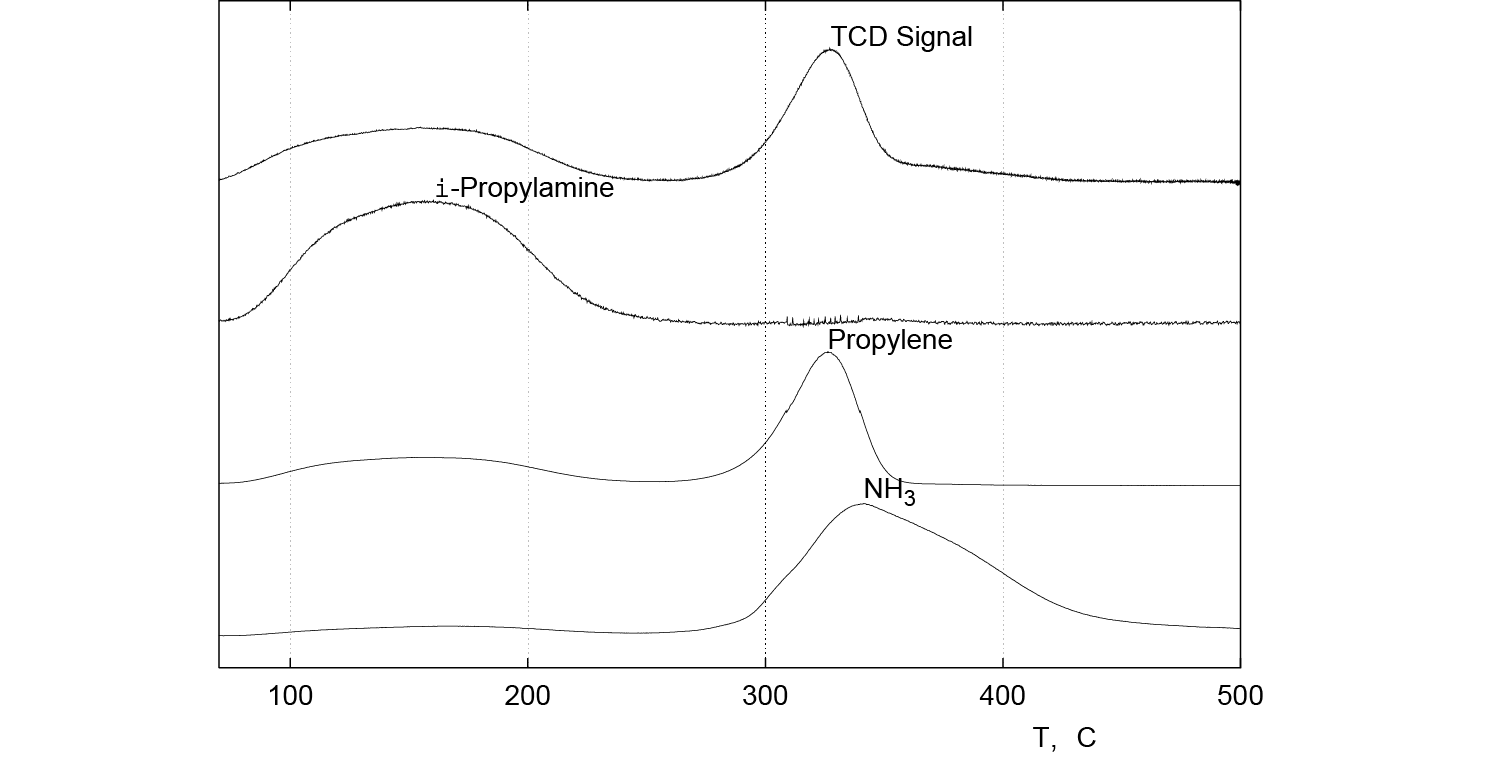
The temperature-programmed decomposition of i-propyl amine should be monitored by a mass spectrometer (Figure 3). At temperatures less than 300 °C, the TPD product contains i-propyl amine. As the temperature exceeds 300 °C, the i-propyl amine is fully desorbed and the TPD products are propylene and ammonia from the Hoffman reaction. In Figure 3, it is clear that only the decomposition products desorb above 300 °C. It is also interesting to note the ammonia desorption lags the propylene desorption. This is due to the readsorption of ammonia onto the ZSM-5. The quantity of the propylene desorbed is then used to calculate the number of acid sites.
Tips for Handling Amines
- Pyridine should be transferred to the vapor generator flask in a fume hood.
- To reduce the stench of pyridine – chill the vapor generator flask in ice for 30 minutes. After filling the flask with pyridine, return the flask to an ice bath to reduce the vapor pressure.
- Consult the MSDS of pyridine for proper handling and exposure limits.
- Purge the vapor valve (not the vapor generator) for 30 minutes after dosing amines. The vapor valve zone should be set to 110 °C during the inert purge.
References
- R. J. Gorte and D. White, Topics in Catalysis, 4 (1997) 57
- A.I. Biaglow, C. Gittleman, R.J. Gorte, R.J. Madon, Journal of Catalysis, 129 (1991) 88
- A.I. Biaglow, D.J. Parrillo, G.T. Kokotailo, R.J. Gorte, Journal of Catalysis, 148 (1994) 213
- T.J. Gricus Kofke, R.J. Gorte, W.E. Farneth, Journal of Catalysis, 114 (1988) 34
- T.J. Gricus Kofke, R.J. Gorte, G.T. Kokotailo, W.E. Farneth, Journal of Catalysis, 115 (1989) 265
- W.O. Haag, Studies in Surface Science and Catalysis, 84 (1994) 1375.
- D.H. Olson, W.O. Haag, R.M. Lago, Journal of Catalysis, 61 (1980) 390
- W.O. Haag, N.Y.Chen, Catalysis Design: Progress and Prospectives, L.L.Hegedus, Ed., Wiley, New York (1987) 181
- J. Tittensor, R.J. Gorte, D. Chapman, Journal of Catalysis, 138 (1992) 714
- E.P. Parry, Journal of Catalysis, 2 (1963) 371
- J.L. Woolery, G.H. Kuehl, H.C. Timken, A.W. Chester, J.C. Vartuli, Zeolites, 19 (1997) 288