Magnesium stearate is widely used in the pharmaceutical industry as an excipient in tableted drugs. The physical properties of the magnesium stearate influence the tableting characteristics of the formulation as well as the in vivo degradation and dissolution of the solid drug. The United States Pharmacopia, which has already established a standard for the particle size distribution of magnesium stearate, may adopt surface area standards for this material in the near future. To assist in this endeavor, Micromeritics obtained four samples of commercially available magnesium stearate and measured the BET surface area using the Micromeritics Gemini Surface Area Analyzer.
Sample Preparation (Degas)
Before a sample can be analyzed, it is necessary to remove gas and vapors which may have adsorbed onto the surface from the ambient air. If this is not done, the surface area result can be low and non-reproducible since an indeterminate amount of the surface will be covered with these materials. This step must be done with caution; every effort must be made not to change the original surface of the sample. This sample preconditioning is usually accomplished by either applying a vacuum or purging the sample with an inert flowing gas.
Both methods usually make use of elevated temperatures to hasten the rate at which the contaminants leave the surface. Caution must be used when heating magnesium stearate because melting, dehydration, sintering, and decomposition are processes that can drastically alter the surface properties of the sample. A test protocol used to establish that excessive sample preparation temperatures have not been used is to determine the surface area of the sample at successively higher preparation temperatures. Duplication of the results at different temperatures indicates that the initial preparation conditions were satisfactory unless both analyses were performed on a completely degraded sample.
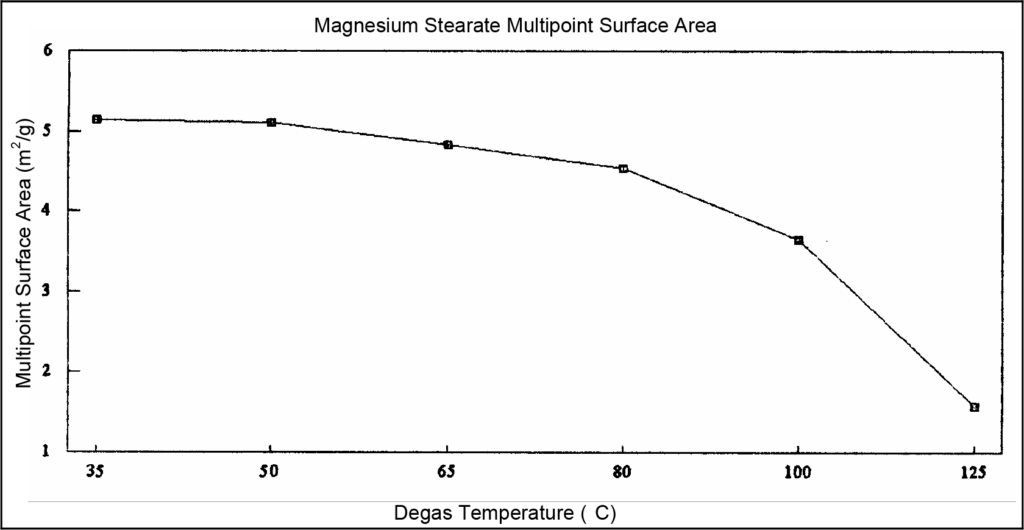
An example of this sample preparation protocol is seen on the following page. One sample was prepared or degassed for four hours at the temperatures indicated in Table 1. The resulting surface areas are listed to the right of the degassing temperatures. These data are shown in graphical format below the table.
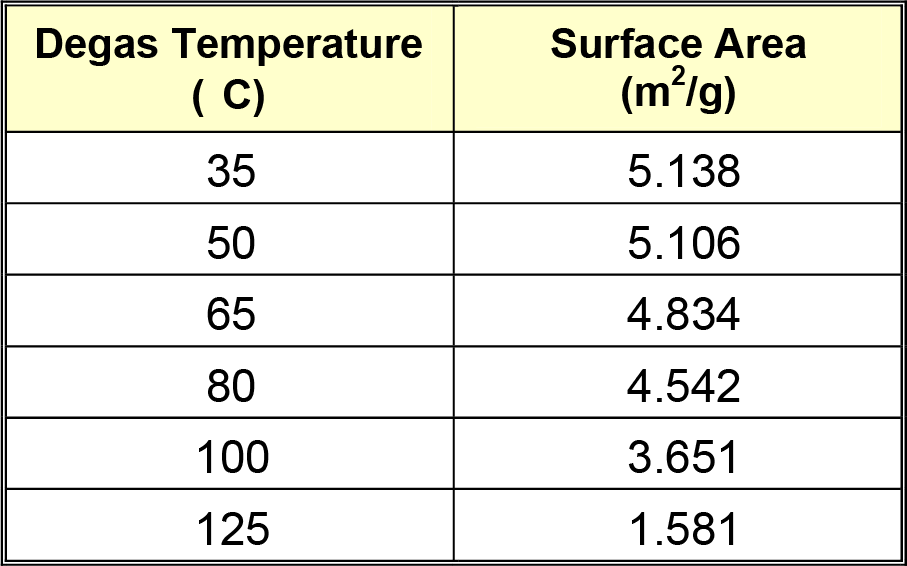
As shown in the above data, magnesium stearate should not be degassed at temperatures above 35 °C because melting and sintering, which lower the measured surface area, occur at higher temperatures.
Analysis of Commercial Magnesium Stearates
Once the sample preparation protocol for magnesium stearate was established, the surface area of four commercially available magnesium stearates was determined. They were degassed for four hours at 35 °C, then analyzed using two methods of analysis for comparison. First the more rigorous multipoint analysis was performed and then, for comparison purposes, a single-point analysis of the same samples, under the same testing conditions, was performed. These results are shown in Table 2. The middle column labeled BET C is indicative of surface energetics.
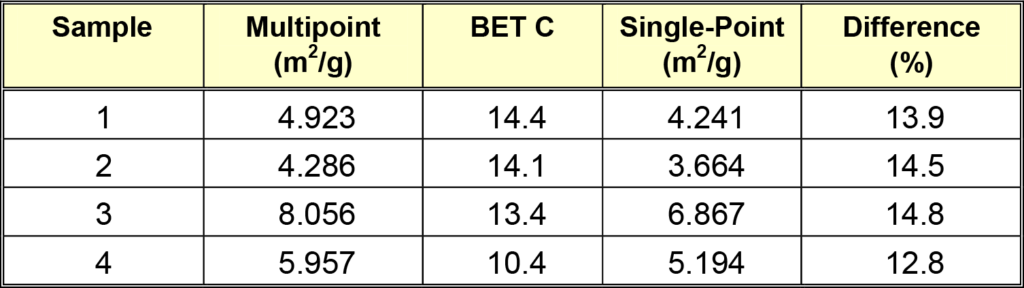
The multipoint surface area values were calculated from data taken at six relative pressure points (P/P0) equal to 0.05, 0.10, 0.15, 0.20, 0.25 and 0.30. The single-point data were calculated at P/P0 equal to 0.30. The regression value for the multipoint BET data yielded a correlation coefficient of 0.9998 or greater for all samples. Also, all multipoint analyses were completed in less than 25 minutes.
The Difference column in the table above was calculated by subtracting the single-point value from the multipoint value and determining its percentage of the multipoint value. It illustrates the magnitude of the error associated with estimating the surface area by using the single-point technique. This magnitude of error is expected for BET C values as low as determined here. In general, BET C values are between 5 and 20 for organic materials. The error in the single-point analysis of these materials is too large for accurate surface data comparison to be made between laboratories or investigators.
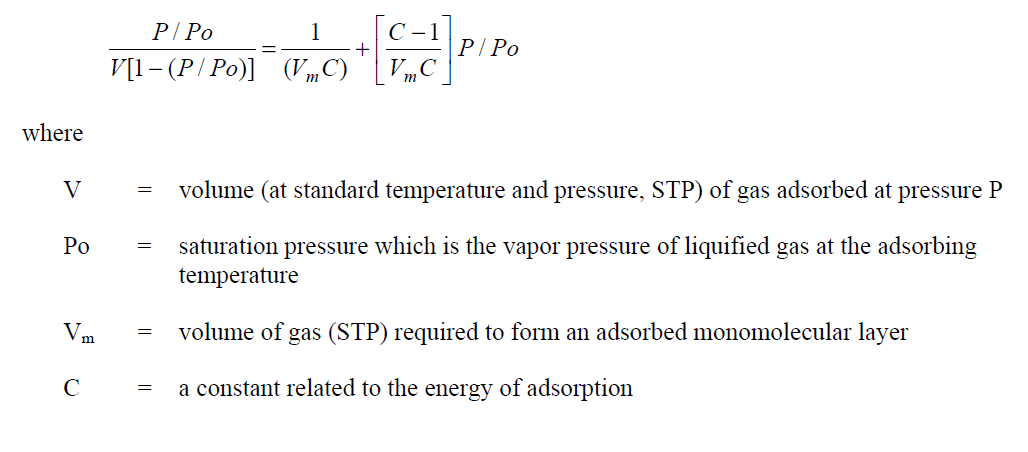
One form of the well-known BET equation that describes the adsorption of a gas upon a solid surface is:
The BET single-point model is derived by assuming the BET intercept equals zero. This approximation becomes valid as the BET C constant approaches infinity. As C → ∞ the BET equation reduces to:
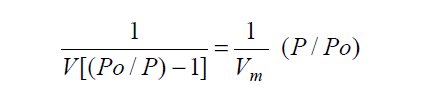
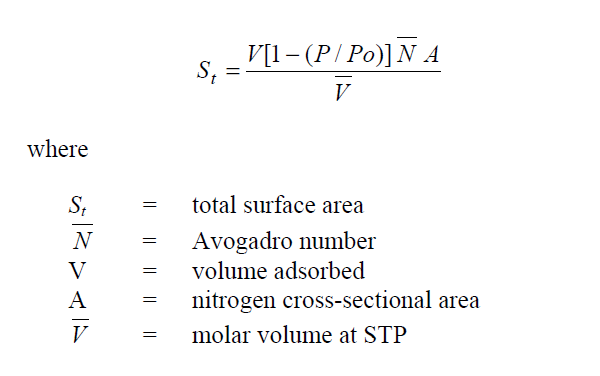
and the total surface area as measured by the single-point method becomes:
The magnitude of the error introduced by the single-point assumption can be calculated by:
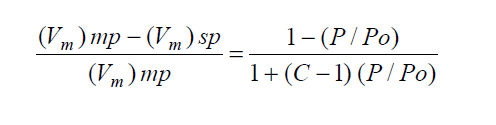
The single-point assumption produces small errors only for high BET C materials, but causes large errors for low BET C materials such as magnesium stearate. The slight gain in analysis speed is thus not justified, especially when the speed and accuracy of the Gemini system is taken into account.