Introduction
Chemisorption is a powerful analytical method used to investigate the surface properties of solid materials, particularly catalysts. Unlike physisorption, which involves weak van der Waals interactions, chemisorption involves strong interactions such as covalent or ionic bonds. This interaction is highly specific, often irreversible, and only forms a monolayer. The interaction highly depends on the chemical nature of both of the surface and the adsorbate.
Chemisorption techniques are essential in the field of heterogeneous catalysis, where information such as number, nature, and strength of active sites on a catalyst surface is crucial for optimizing performance. Such information allows scientists to determine metal dispersion, and evaluate the adsorption strength, activity, and reactivity of a catalyst, which are the key parameters in catalyst design and evaluation.
Several chemisorption techniques have been extensively utilized to evaluate catalysts, including pulse chemisorption and temperature-programmed analyses such as reduction, oxidation, desorption, decomposition, and surface reactions. In this application note, pulse chemisorption technique will be applied to Micromeritics reference standard material to demonstrate its utility in catalyst characterization on the ChemiSorb Auto.
Pulse Chemisorption Working Principle
In pulse chemisorption technique, a flow of hydrogen in argon is introduced to the sample bed to reduce the active metal at an elevated temperature. Then, an inert gas flows through the sample bed to remove any residue of the reductant at elevated temperature. The sample is cooled to room temperature. Finally, the technique works by dosing a known amount of probe gas such as hydrogen (H2), carbon monoxide (CO), oxygen (O2), or nitrous oxide (N2O) depending on the type of active metals.
The system will dose until the sample is saturated with the adsorbate gas. Depending on the amount of probe gas being adsorbed, any unreacted gas will reach the thermal conductivity detector (TCD) appearing as peaks in the detector signal. The calibrated loop precisely injects a known quantity of adsorbate into the sample bed.
Selection of Adsorbate
Pulse chemisorption is a surface characterization technique widely used to quantify the number of active sites available, metal dispersion, for chemical reactions on a solid material. It is also used to study the active metallic surface area in some applications. However, the selection of an appropriate adsorbate is critical and should be carefully considered based on two key factors, stoichiometric factor and binding affinity.
For metals such as copper and silver, the binding affinity for H2 and CO is negligible, resulting in little to no adsorption. However, when N2O is introduced as an adsorbate, it exhibits strong binding affinity with these metals, making it a more suitable choice for chemisorption analysis.
Oxygen is commonly used in pulse chemisorption for hydrogen-oxygen titration. In the case of palladium (Pd), hydrogen tends to form hydride with the Pd. Therefore, CO is often preferred for Pd-based catalysts. However, using hydrogen can also be problematic when the catalyst is supported on carbon, as the support itself can significantly adsorb hydrogen, leading to inaccurate measurements.
For platinum (Pt), both H2 and CO can be used in pulse chemisorption experiments, as each can adsorb onto the metal surface. However, the choice of adsorbate affects the stoichiometric factor used in metal dispersion calculations. Hydrogen binds dissociatively to Pt, resulting in a stoichiometric factor of 2. In contrast, CO can bind in either a linear or bridged fashion, each with different stoichiometric factors. For the Pt/Al2O3 standard material, CO binds in a linear fashion, which corresponds to a stoichiometric factor of 1.
Results and Discussion
For this type of analysis, 0.5% platinum alumina has been evaluated on the ChemiSorb Auto using both H2 and CO as a probe gas with a metal dispersion specification of 31% ±5%. Figures 1A and 1B show pulse chemisorption profiles using 10% H2/Ar and 10% CO/He as probe gases, respectively. The thermal conductivities of H2 and Ar relative to air are 7.07, and 0.68, respectively. This significant difference allows the TCD to effectively distinguish the unreacted H2 signal.
In some cases where 10% H2/Ar blend is not available, nitrogen may be used as an alternative carrier gas. Given that the thermal conductivity of N2 relative to air is 1.00, a 10% H2/N2 mixture is also suitable for pulse chemisorption applications.
In experiment one, using 10% H2/Ar as the adsorbate (Figure 1A), the first peak was fully consumed by the sample; an ideal scenario indicating complete adsorption. The second peak corresponded to the partial saturation stage, where most of the gas exited the sample tube and reached the TCD. The final three peaks indicated that the sample was saturated with hydrogen gas; hence, further injections were unnecessary.
Similarly, in Experiment Two, where a 10% CO/He blend was used as the adsorbate (Figure 1B), the first peak indicated complete consumption of the adsorbate by the active metal. The second and third peaks corresponded to a partial saturation stage. The instrument stopped injecting gas into the system once the difference between consecutive peak areas was within 5%.
By integrating the peak areas and calculating the cumulative amount of gas adsorbed. Metal dispersion, metallic surface area, and crystallite size information can be obtained. Six analyses of this particular batch of standard material have been done on the same ChemiSorb Auto model. For these 6 analyses, the average metal dispersion and standard deviation have been reported in Table 1.
Table 1. Repeatability results for six analyses on 0.5% platinum alumina using CO and H2 as probe gases on ChemiSorb Auto model.
| 0.5% Pt-Al | RUN 1 | RUN 2 | RUN 3 | x̄ | σ |
| Metal Dispersion (%), CO | 31.88 | 32.22 | 30.06 | 31.39 | 1.16 |
| Metal Dispersion (%), H2 | 34.73 | 34.21 | 34.94 | 34.63 | 0.37 |
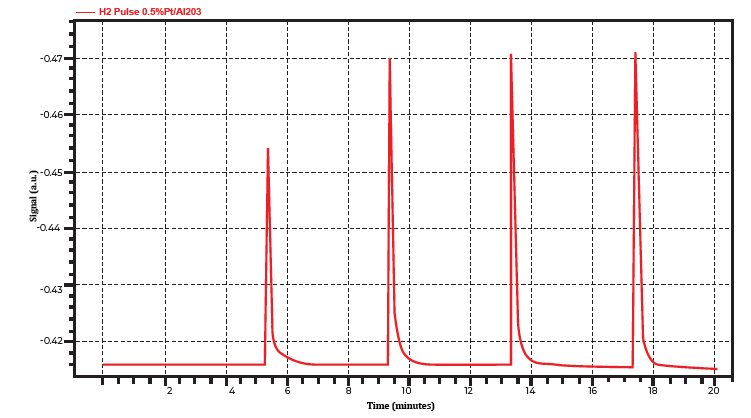
Figure 1A. A typical profile with one fully consumed, one partially consumed, and three saturation peaks using 10% H2/Ar as a probe gas on 0.5% platinum alumina.
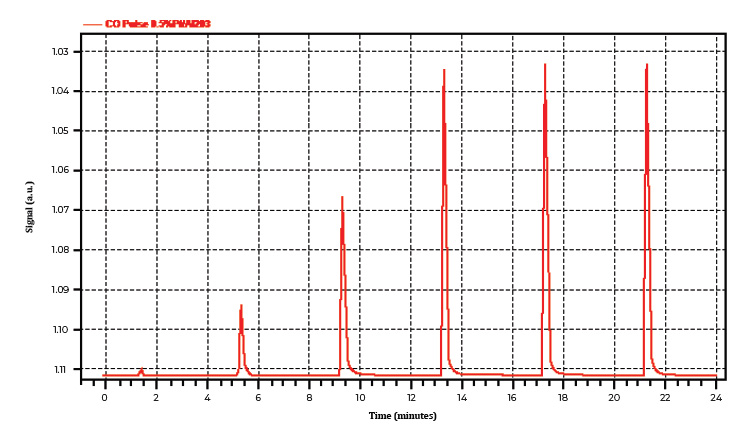
Figure 1B. A typical profile with one fully consumed, two partially consumed, and three saturation peaks using 10% CO/He as a probe gas on 0.5% platinum alumina.
A 0.5% Pt/Al2O3 metal loading does not imply that all of the platinum is actively participating in chemical reactions. Therefore, measuring metal dispersion is essential to evaluate the activity of a catalyst. For instance, in Experiment One pulse chemisorption yielded a dispersion of 31.39%, indicating that only 31.39% of the platinum is accessible and actively involved in surface reactions. The remaining platinum may be embedded within the bulk material or trapped inside the support structure, making it inaccessible for catalytic activity.
The method of catalyst preparation plays significant role in determining accessibility. In some cases, active metal particles may become embedded within the support, thereby blocking some of the active sites.
The ChemiSorb Auto is a unique instrument capable of yielding valuable data on the percentage of active species present on the catalyst surface. A higher metal dispersion value generally correlates with greater catalytic activity. Understanding this activity helps scientists make informed decisions about scaling up production of desired products or redesigning catalysts to improve performance.