Helium is the most used gas for pycnometry due to its ideal behavior; However, there are times where helium can be substituted for other gases. Helium has the ability to permeate into pores that are closed from the surface and interacts with some organic materials and microporous carbons. Nitrogen is the second most common gas, but also interacts with certain materials. Larger molecules such as sulfur hexafluoride and methane can be used to include the volume of very small pores in volume results from which density is being calculated.
Determination of skeletal density is obtained by dividing the sample’s mass by the skeletal volume. The equation to determine skeletal volume by the 10 cm3 AccuPyc is given by:
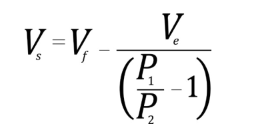
Where Vf is the filling volume and Ve is the expansion volume, which are measured during calibration. These values are unique to the selected gas and remain constant while using this gas, temperature, and filling pressure combination. The AccuPyc pressurizes the sample chamber to a specified pressure. The gas equilibrates and P2 is determined. The expansion valve, located between the sample and expansion chambers, is opened and the gas located in the sample chamber flows into the expansion chamber, causing the pressure to decrease. When the gas equilibrates, P2 is determined.
Gas interacting with a material during the determination of P1 causes the pressure reading to decrease, possibly resulting in a negative sample volume. This interaction is typically either adsorption or permeation.
Pressure is required to reach equilibrium before collecting P1 or P2 data. The default equilibration rate is 0.005 psig/min and was initially used for all samples. If the sample could not equilibrate with the default rate, then a rate was selected based on the monitored pressure change. Reasons why equilibration rates might not be obtainable are due to diffusion, gas interacting with the material, material outgassing, or vapor pressures caused by liquids.
Seven different gases were used to analyze seven materials. The gases used were helium, nitrogen, argon, carbon dioxide, dry air, sulfur hexafluoride, and methane. The samples analyzed with these gases were metal spheres, alumina, battery separator, carbon black, 5A, ibuprofen, and water.
Metal Spheres
The metal spheres are a non-porous tungsten carbide alloy, and are used for calibration of the pycnometer filing and expansion volumes. Results of the analyses using the different gases are shown in Table 1. The only notable difference is the carbon dioxide analysis length was longer.

Alumina
Alumina catalyst support results are shown in Table 2. Measurements with carbon dioxide and sulfur hexafluoride resulted in negative volumes. Density is not calculated when the measured volume is negative. Such analyses are indicated with N/A in the tables. Measuring negative volumes is explained further down. Density results for remaining gases are higher than helium, possibly due to interaction with the sample.

Battery Separator
Celgard H1612 16 µm trilayer microporous membrane results are shown in Table 3. The carbon dioxide, sulfur hexafluoride, and methane appear to have interacted with the material, causing a higher density value.

Carbon Black
Carbon dioxide, sulfur hexafluoride, and methane appear to have interacted with the sample.

5A
5A molecular sieve results are shown in Table 5. Helium and sulfur hexafluoride were the only gases that successfully ran while the other gases measured negative volumes. The small helium molecules were able to access the small pores in the material and produce reasonable results while the very high sulfur hexafluoride results indicated a problem with the analysis in that the measured volume was very small, though not quite negative.

Ibuprofen
Generic brand ibuprofen results are shown in Table 6. Results are similar between all of the gases.

Water
Deionized water filtered to 0.1 µm results are shown in Table 7. Results are similar between all of the gases.

Obtaining Negative Volumes
The 10 cm3 AccuPyc pressurizes the sample chamber first, then allows the gas to expand into the expansion chamber after pressure equilibration has been reached. When inert gases are used, the initial pressure (P1) reading is very close to the entered filling pressure. ‘The expansion valve opens, the gas equilibrates between the two chambers, and the pressure decreases. The final pressure (P2) reading is collected after equilibration.
When gases interact with a material, the pressure reading for P1 is lower than expected due to the gas slowly adsorbing on to the material before the pressure can equilibrate. When the expansion valve opens, the gas equilibrates between the two chambers. The increased volume added by the second chamber causes the pressure to decrease. The gas that adsorbed begins to desorb. This causes P2 to be higher than expected. These two unexpected values for the pressure readings cause the volume to be negative.
Pressure readings for helium and nitrogen on 5A can be compared to show the differences in pressure values. A filling pressure of 19.5 psig was used. The measured P1 values for helium is 19.756 psig, while nitrogen is 18.906 psig. For , the helium value is 10.350 psig and the nitrogen is 15.367 psig. The volume obtained with helium is 2.5245 cm3 and nitrogen is -24.6783 cm3.
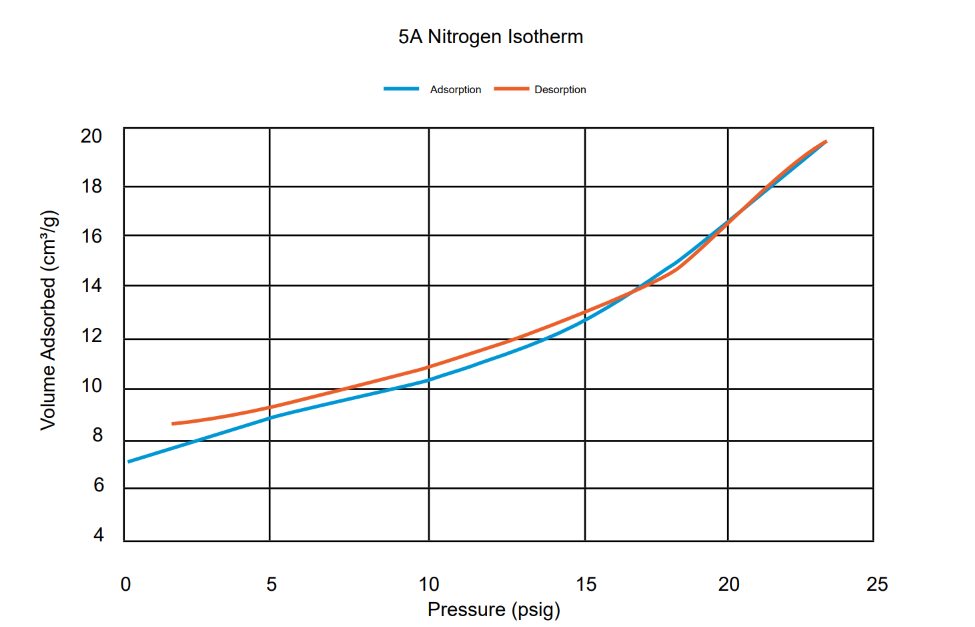
To prove this effect is coming from adsorption, a nitrogen analysis at room temperature was performed on the 5A sample, using the High Pressure Volumetric Analyzer (HPVA). The measured isotherm is shown on Plot 1. It is clear that nitrogen adsorbs and desorbs under the conditions previously described.
Conclusion
Gas selection does not appear to be significant if the selected gas is capable of entering the pores of interest and does not interact with the material. This is proven based on data obtained using the various gases for the metal spheres, ibuprofen, and water samples. Since this does not apply to the majority of materials, care should be taken when determining a gas to use.