Introduction
With the release of the MicroActive V6.0 and the 3Flex V6.0 software, multicomponentadsorption predictions using ideal adsorbed solution theory (IAST) have been added. IAST is apredictive technique developed by Myers and Prausnitz in 1965. It is used to predict mixed gasadsorption behavior from single component isotherms. This first release of IAST on V6.0 canbe used to predict binary adsorption. IAST has proven to be in good agreement for a variety ofbinary systems, including methane/ethane mixtures in BPL carbon, Xe/Kr mixtures in zeoliteNaA, and propane/propylene mixtures in HKUST-1 (Furmaniak, et. al., pccp, 2015).
IAST relies on several assumptions that are described in the original manuscript, which are listed
below (Myers, Prausnitz, AIChE Journal, 1965).
- The surface of the adsorbent is heterogeneous. Homogeneous surfaces are likely to deviate from ideal behavior.
- The adsorbents are non-reactive and similar in size/shape to each other.
- Pure-component isotherms must be accurately measured at low surface coverage, because the integration for spreading pressure is sensitive to this portion of the pure-component isotherm.
Experimental
IAST calculations can be performed in MicroActive by selecting Reports then OpenNotebook. If creating a new notebook, enter a name and press Open. The system will displaya prompt that the chosen file name does not exist and ask whether to create that file. Afterproceeding with the file creation, the report selection screen will appear where IAST CompositionReports can be selected to open the IAST report template. On the IAST reporting window, up totwo isotherms for IAST can be selected. The following isotherm models are also available: VTTE,Sips, Langmuir, Dual Site Langmuir, Toth, and Redlich Peterson. Choose the appropriate model,click Save, then press Preview to generate the report.In this note, three microporous carbons were selected for demonstrating the new IAST featuresin MicroActive. The three carbons used in this study include: Carboxen 1018, Carboxen 1021, andCarbosieve S-III. The samples were each analyzed on the 3Flex for CO2, CH4, and C2H6 adsorption.Prior to analysis, samples were activated on a SmartVac Prep by heating to 250 °C under vacuumfor 10 hours. Following activation, the samples were analyzed for each gas.Breakthrough analyses were conducted on the Micromeritics BTA. Samples were activatedunder nitrogen flow while heating to 250 °C overnight. Binary breakthrough measurementswere conducted using a mixed gas feed consisting of 50:50 mixtures of CO2-CH4, CO2-C2H6, andCH4-C2H6. Nitrogen was used as a carrier gas and argon was used as a tracer gas to determinethe start of the breakthrough experiment
Results
Single Component Analysis using the 3Flex
The results of the single component isotherms for the three materials: Carboxen 1018, Carboxen1021, and Carbosieve S-III are shown in Figures 1, 2, and 3 below.
The results for Carboxen 1018 are shown in Figure 1. Ethane showed strong affinity at low pressure, but less capacity than CO2 at 1000 mbar. At 1000 mbar, the adsorption capacity of CO2 was 55 cm3/g STP, while ethane reached a capacity of 40 cm3/g STP. Methane was the weakest adsorbing species, reaching a capacity of 15 cm3/g STP.
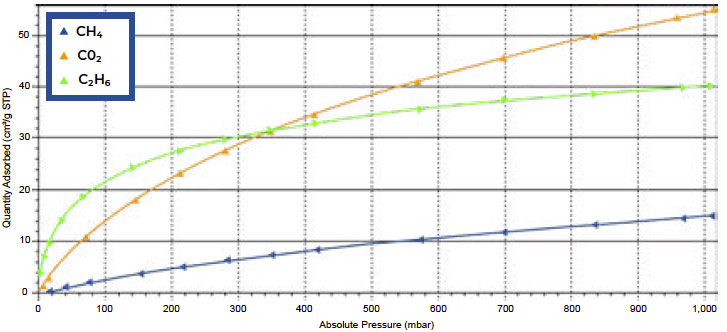
Figure 1. Single component isotherms of Carboxen 1018 for CO2 (orange), CH4 (blue), and C2H6 (green)
Carboxen 1021 displayed a similar trend compared to Carboxen 1018; however, the adsorbedcapacity at 1000 mbar was similar between ethane (58 cm3/g STP) and CO2 (55 cm3/g STP). Onceagain, methane adsorption was the lowest at 1000 mbar, reaching only 24 cm3/g STP.
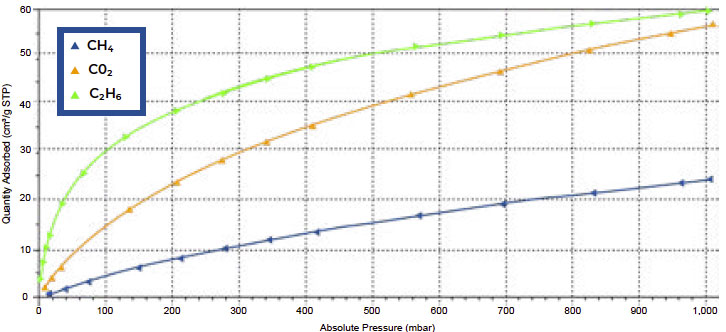
Figure 2. Single component isotherms of Carboxen 1021 for CO2 (orange), CH4 (blue), and C2H6 (green)
Carbosieve S-III showed the strongest affinity for ethane, reaching an adsorbed capacity of 90 cm3/g STP at 1000 mbar. CO2 adsorbed the second most (74 cm3/g STP), followed by methane (33 cm3/g STP).