Micromeritics produces several instruments used for determining physical adsorption isotherms. Most of these utilize the static (manometric) adsorption method, where the amount of gas adsorbed by the sample is determined from pressure and temperature measurements, and gas laws that account for the non-ideal behavior of the adsorptive. The amount of gas dosed onto the sample from a gas manifold is determined using the calibrated volume of the manifold, the absolute gas pressure in the manifold, and the manifold temperature. Pressure and temperature are recorded first before the dose to determine the initial amount of gas in the manifold, and after the dose to determine the amount of gas that remains in the manifold. The difference is the amount of gas that has moved from the manifold into the sample holder, or the amount of gas dosed onto the sample.
The adsorptive that moves into the sample holder can do one of two things; it can either adsorb onto the sample or remain in the gas phase above the sample. It is that part that remains in the gas phase that establishes the pressure in the sample holder as pressure can be thought of as a measure of gas concentration. The adsorption of gas on the surface and into pores can be thought of as a concentration of gas on the sample surface as a result of physical attractive forces between the sample and the adsorptive.
In order to determine the amount of gas that adsorbs onto the sample, the dependent variable of the isotherm, it is necessary to separate the amount of gas dosed into the sample holder into these two portions, the amount that remains in the gas phase, and contributes to pressure, and the amount that adsorbs onto the sample. Gas laws can be used again to determine the amount of gas that does not adsorb. To account for small differences in cryogenic bath temperatures, results are reported as a function of pressure relative to that of the saturation vapor pressure of the adsorptive at the bath temperature. All that is needed is the volume of the sample holder, corrected for the temperatures of the various spaces within it. The gas capacity of the sample holder is often called the free space, or void space. This capacity increases as the temperature in the sample holder decreases, and so is a function of the bath temperature. In addition, the amount of gas within the portion of the sample holder that is at the bath temperature needs to be corrected for compressibility, or non-ideal behavior. For nitrogen at its normal boiling point, 77.35 K, this accounts for an increase in amount of nitrogen in the container of about 4.3% per atmosphere above what would be calculated from the free space volume alone.
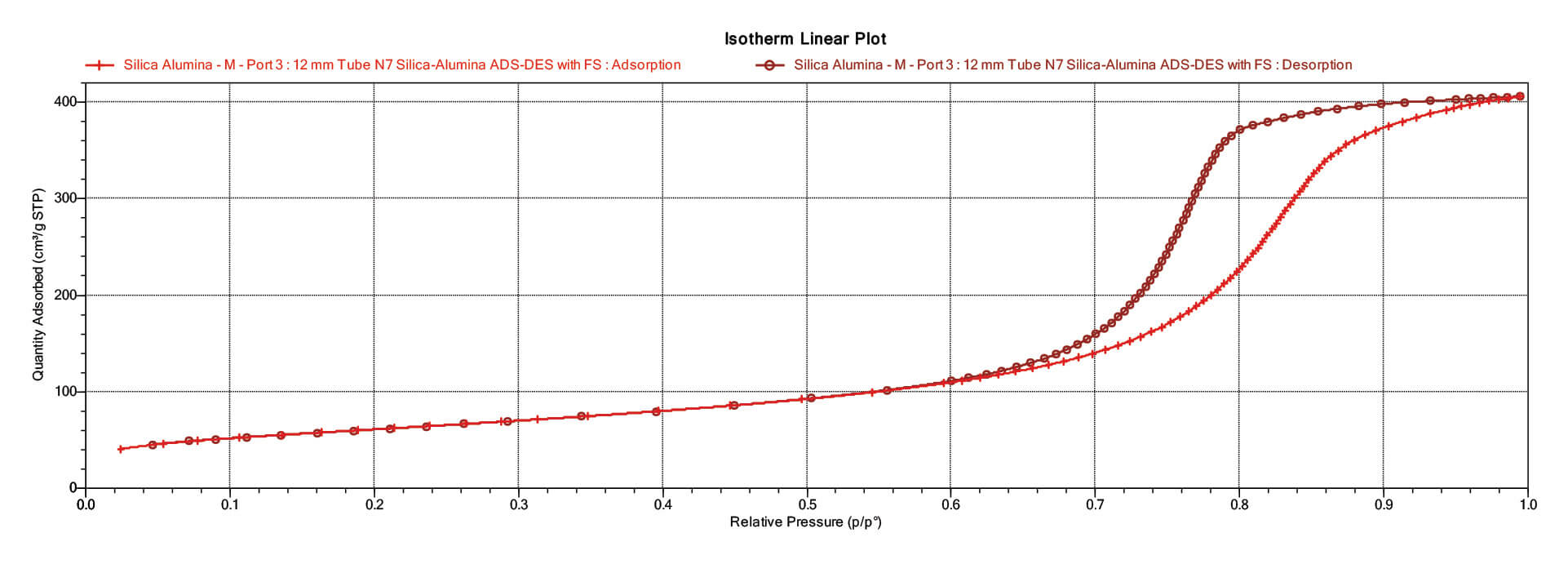
Obviously, the amount of adsorptive dosed into the sample holder that is not in the gas phase is the amount of gas that adsorbed onto the sample surface or within its open pores. In order to calculate this amount adsorbed, then, accurate values of the free space must be known, both that at the temperature of the bath, and that remaining at ambient temperature, including the portion of the sample holder that is outside the Dewar flask that contains the cryogen. Thus, two free spaces values are needed, that determined with gas laws when the tube is at ambient temperature, and then that determined when a portion of the tube is immersed in the bath. These two free space values allow separation of the free space volume into that remaining at ambient temperature, and that at the bath temperature. This allows for determination of the amount of adsorptive in the gas phase in the full empty volume of the sample holder, including correction for compressibility for that portion at cryogenic temperature. These two determined free space values are sometimes referred to as cold and warm free space, indicating the temperature of the sample when determined, and sometimes referred to as analysis and ambient free space, again referring to the temperature of the sample when the free space determination is made. Warm or ambient free space is determined with the sample holder at ambient temperature; cold or analysis free space is determined with a portion of the sample holder at the analysis temperature. Figure 1 shows the adsorption and desorption isotherms determined for an alumina catalyst support extrudate.