Introduction
Chemisorption is a critical analytical technique used to investigate the surface properties of solid materials, particularly in the field of catalysis. Unlike physisorption, which involves weak van der Waals forces and is typically reversible, chemisorption involves strong, specific interactions such as covalent or ionic bonding. These interactions often result in the formation of a monolayer and are generally irreversible, making chemisorption highly selective and informative for surface characterization.
While physisorption is commonly used to determine surface area and pore structure, chemisorption techniques provide critical insights into the number, nature, and strength of active sites on catalyst surfaces. This information is essential for evaluating metal dispersion, adsorption strength, and catalytic reactivity; key parameters in catalyst design and performance optimization.
Temperature-Programmed Reduction Working Principle
Temperature-programmed reduction (TPR) is considered the most commonly used chemisorption technique for characterizing the reducibility of metal oxides. It yields information such as oxidation states of the metal oxides and the strength of metal-support interactions. Knowing at what temperature the metal oxide is being fully reduced provides information about catalyst activation. In a TPR profile, the number of reduction peaks correspond to the oxidation states. The area under the peak can be used to quantify the amount of hydrogen consumption.
The working principle of temperature-programmed reduction is quite simple. A 10% mixture of H2/ Ar gas flows through the sample bed as the temperature is increasing linearly. Hydrogen reduces metal oxides to metals and produces water. A slush bath, mixture between liquid nitrogen (LN2) and isopropyl-alcohol (IPA), was utilized to trap moisture from the reaction. Using copper oxide as an example, copper oxide reacts with hydrogen to produce copper and water as a result. Figure 1 shows a copper oxide TPR profile.
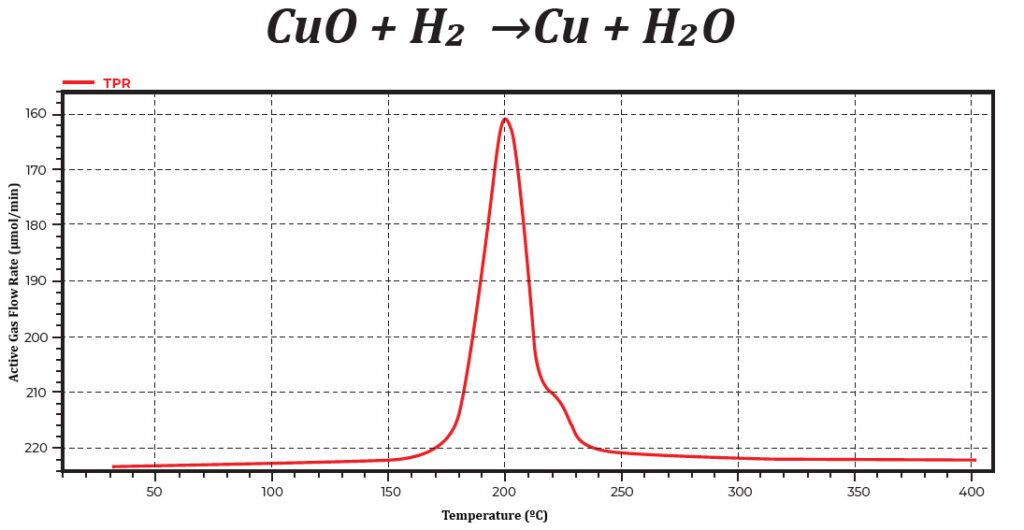
Figure 1. Temperature-programmed reduction profile of copper oxide. The active gas concentration is shown as a function of temperature.
Three analyses were conducted using ChemiSorb Auto. The results were within the specification
indicated in the standard material booklet. Table 1 displays the average and standard deviation
of those analyses.
| CuO | RUN 1 | RUN 2 | RUN 3 | x̄ | σ |
| Peak Temp (°C) | 195.90 | 195.70 | 200.00 | 197.20 | 2.43 |
| H2 Consumption (cc/g) | 27.79 | 29.32 | 30.04 | 29.05 | 1.15 |
Mechanism of Reduction
The shape of the peak provides information about the particle size. There are two reduction mechanisms [1] (Figure 2) relevant to interpreting TPR profiles. In the case of very small and fine particles, H2 can rapidly initiate reduction by forming the first nuclei. This process occurs quickly, resulting in a sharp and symmetric peak in the TPR profile. This type of behavior is characteristic of the nucleation mechanism.
In other cases, larger catalyst particles may be present. When H2 gas flows over the surface as temperature increases linearly, reduction begins at the outer shell of the particle. H2 must then diffuse inward to reach the subsequent layers, which introduces a diffusion limitation and results in a slower overall reduction process.
This phenomenon typically manifests as a broader and larger peak in the TPR profile, which is potentially followed by a shift in the reduction temperature. This type of behavior is described by the “contracting sphere” model.
By analyzing the shape of TPR profile, one can infer qualitative information about particle size. In catalytic applications, the ideal scenario involves small, finely dispersed particles on the surface of the support, maximizing their accessibility and reactivity in chemical reactions.
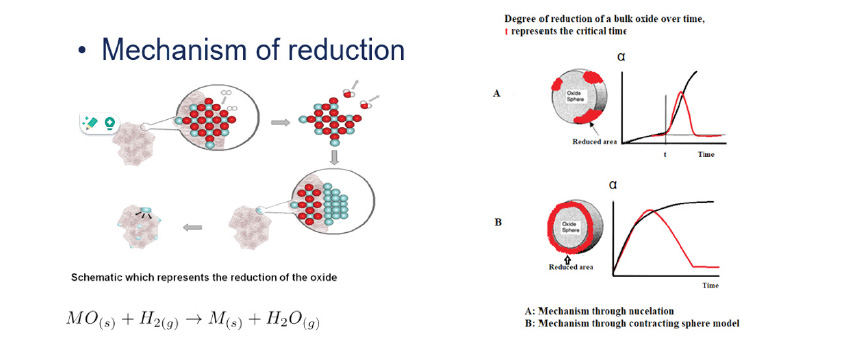
Figure 2. Particle size reduction mechanism models.
Effect of Promoters
TPR offers both quantitative data and qualitative insights into catalyst activation behavior. The appearance of a reduction peak in the TPR profile is commonly interpreted as an indicator of catalyst activation. However, the reduction temperature itself provides critical information that should be considered during catalyst design.
If a finely dispersed catalyst requires a high temperature for activation, there is an increased risk of sintering. Sintering leads to a reduction in the metallic active surface area, which reduces the number of available active sites for chemical reactions. This process is non-spontaneous and typically results in a loss of catalytic activity.
For instance, Figure 3 demonstrates a shift in the reduction temperature of CuO as a function of palladium (Pd) loading. As shown, increasing the Pd content lowers the reduction temperature, which is considered favorable. Activating a catalyst at lower temperature helps preserve dispersion and mitigate the risk of sintering.
The key takeaway is that TPR is an essential tool in catalyst characterization. It enables scientists to evaluate how effectively the support stabilizes the active species under elevated temperature and pressure conditions.
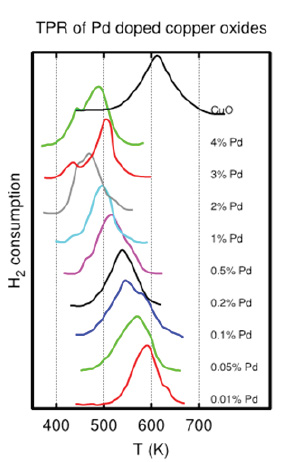
Figure 3. Effect of different loading of promoters on CuO.
Automatic Gas Calibration
The ChemiSorb Auto features a patented blend valve that allows users to automatically perform gas calibrations. A gas calibration is required to quantify the quantity consumption of H2. This calibration was done automatically by blending pure argon and pure hydrogen, adjusting the ratio of hydrogen from 10% to 0% in an eleven step process. Figure 4 displays a H2/Ar gas calibration profile.
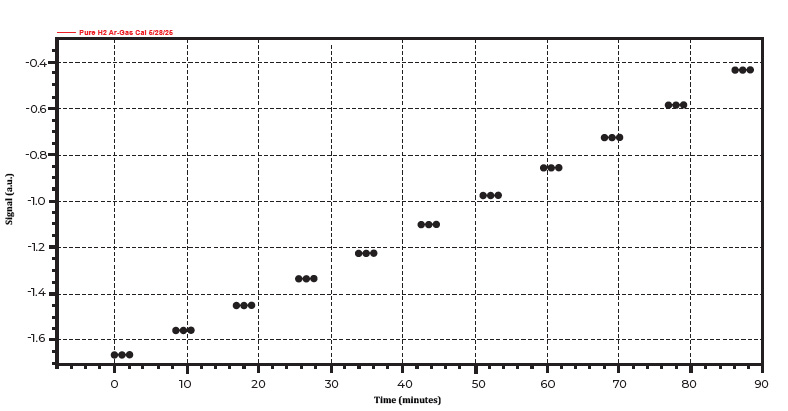
Figure 4. A typical gas calibration profile for TPR experiment.
Gas calibration can be performed either before or after the analysis. A new calibration is not
required for every new analysis, unless the analysis conditions such as gas concentrations or
flow rates differ from those used in the previously established calibration. In such cases, a new
calibration ensures accuracy and consistency under the updated conditions.
Reference
Webb, P; Orr, C; Yunes, S. Analytical. Med. Tech, 1st ed. Micromeritics Instrument
Corp, 1997; pp 232-234.