Introduction
Separation processes are a critical component of the chemical industry. Olefin/Paraffin separations are one of the most industrially relevant separations for the production of plastics. Globally, 150 million metric tons of ethylene and 130 million metric tons of propylene are produced each year. Pressure swing adsorption, membrane separations, and cryogenic distillation are three methods that can be used to separate olefins from paraffins for the production of plastics.
Cryogenic distillation is the most frequently used method for the separation of ethane and ethylene. Cryogenic distillation separates ethane and ethylene by their difference in boiling points. Ethane has a boiling point of -89°C and ethylene has a boiling point of -103.7 °C. Due to the similarity of these boiling points, ethane and ethylene separation by cryogenic distillation is extremely costly, typically requiring hundreds of trays in a distillation column to achieve the necessary purity of ethylene as a product.
Membrane-based separations of ethane and ethylene are performed at smaller scale than cryogenic distillation. Once again ethane and ethylene are similar in size and shape, making the separation difficult. A large pressure gradient is required to successfully separate the two gases. Additionally, the separation performance of a membrane is not capable of achieving the same purity of ethylene that can be produced in a distillation column. Therefore, further processing is generally required to achieve the desired purity.
Adsorptive-based separations, such as pressure swing adsorption, is the final method of ethane and ethylene separation. Pressure swing adsorption requires less energy for separation of ethane from ethylene compared to cryogenic distillation. Like membrane separations, however, it is difficult to achieve the desired purity in one step such that multiple adsorption columns are necessary. Nevertheless, new materials are continually being investigated to improve the adsorptive based separation performance for ethane and ethylene.
Experimental
Molecular sieve 5A was used for ethane-ethylene adsorptive separation experiments. Prior to analysis, MS 5A was activated under helium flow for 1 hour at 100 °C followed by heating at 300 °C for an additional 10 hours. The sample was then allowed to cool to room temperature before breakthrough measurements began.
Breakthrough was conducted at 25 °C and atmospheric pressure using an equimolar flowrate of ethane (2 sccm) and ethylene (2 sccm) in a helium (15 sccm) carrier gas. Argon (1 sccm) was used as a tracer gas to determine the start of the breakthrough measurements. The experiment was considered complete when the measured outlet concentration reached an equilibrium. Following breakthrough, the sample was allowed to purge under helium flow for 20 minutes before heating to 100 °C to remove all adsorbed ethane or ethylene. A total of three breakthrough measurements were conducted in cycles with complete activation prior to each cycle.
Results
The breakthrough curves for the three ethane-ethylene adsorption experiments on molecular sieve 5A are shown in Figure 1 below.

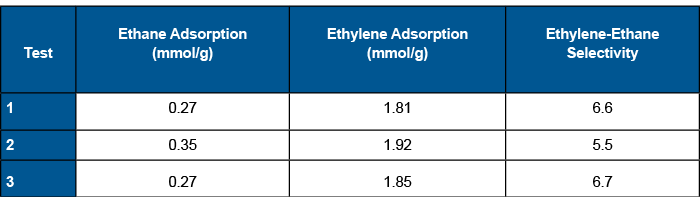
All three measurements were in agreement and display peak shapes that are consistent with competitive adsorption between ethane (weak adsorbent) and ethylene (strong adsorbent). Ethane roll-up is evident in the breakthrough curves and a significant quantity of ethane is displaced by the adsorption of ethylene. The equilibrium quantity of ethane adsorbed was roughly 0.3 mmol/g and the equilibrium quantity of ethylene adsorbed was roughly 1.9 mmol/g resulting in a selectivity between 5.5 – 6.5 across the three measurements.
During the breakthrough experiments, argon (tracer gas) breaks through first, followed by ethane. The argon concentration drops upon breakthrough of ethane because the concentration of the exit gas changes. At the beginning of the experiment, only argon and the carrier gas helium are flowing to the mass spectrometer. Once ethane breaks through, argon, helium, and ethane all reach the mass spectrometer. Initially, the outlet ethane concentration is higher than the feed concentration due to rollup. Ethane concentration drops to the feed concentration after ethylene reaches saturation. At this time, all species have reached equilibrium and the breakthrough experiment is complete. Table 1 below shows the equilibrium quantities adsorbed for ethane and ethylene as well as the selectivity across all three experiments.
Conclusion
Molecular sieve 5A is an effective adsorbent for the separation of ethylene from ethane reaching a selectivity of roughly 6.5 in these breakthrough studies. Additionally, we determined that the ethylene equilibrium adsorption quantity was 1.9 mmol/g.