This application note explains what aluminas are, their use as a vapor trap, and how a BET surface area measurement can be used as an indicator for their performance.
Alumina is more correctly known as aluminum III oxide. Porous aluminas are produced from sintering the material at elevated temperatures; alumina in a pellet form is widely used in science and industry to trap out unwanted water and other vapors. Common industrial applications for alumina vapor traps are compressors and vacuum pumps. Use of these traps allows air or gases at the required mass flow rates to pass through the trap to the device and, importantly, the air is supplied dry and free from vapor contamination.
The alumina manufacturing process allows for some control of the porosity of these beads and their resultant surface area. It is the porosity and surface area characteristics of the alumina bead which makes it useful as a vapor trap. These materials typically have surface areas of hundreds of square meters per gram as measured by the BET surface area method. The large available surface area efficiently traps out vapors from the gas stream and adsorbs or “bonds” them within the alumina.
With time and use, the alumina becomes less efficient as the surface area decreases and porosity is lost. Attrition between the pellets can also lead to their breakdown in the trap, resulting in restricted air flow and loss of efficiency.
Alumina can be easily regenerated by heating in an oven; this renewal process, however, cannot be carried out indefinitely. Characterization of the regenerated material and a comparison of its surface area to virgin material are key to being able to assess on-going use and to choose the right time to start using a new batch of alumina. The surface area of the alumina can be easily determined using the Micromeritics TriStar gas adsorption analyzer and the application of the well-established BET method.
In this technique, the sample is first cleaned of contaminants by the application of heat and evacuation, or a flowing inert gas stream. The sample is analyzed on the instrument at cryogenic temperatures and the quantity of nitrogen gas needed to give monolayer coverage of the available surface is determined by the TriStar.
To accomplish this, an isotherm is collected illustrating how the pressure over the sample varies with the amount of gas taken up by the sample. Collecting the whole isotherm allows us to measure not only the surface area of the sample, but also its pore size distribution up to 300 nm and obtain a total pore volume (TPV) measurement, if required.
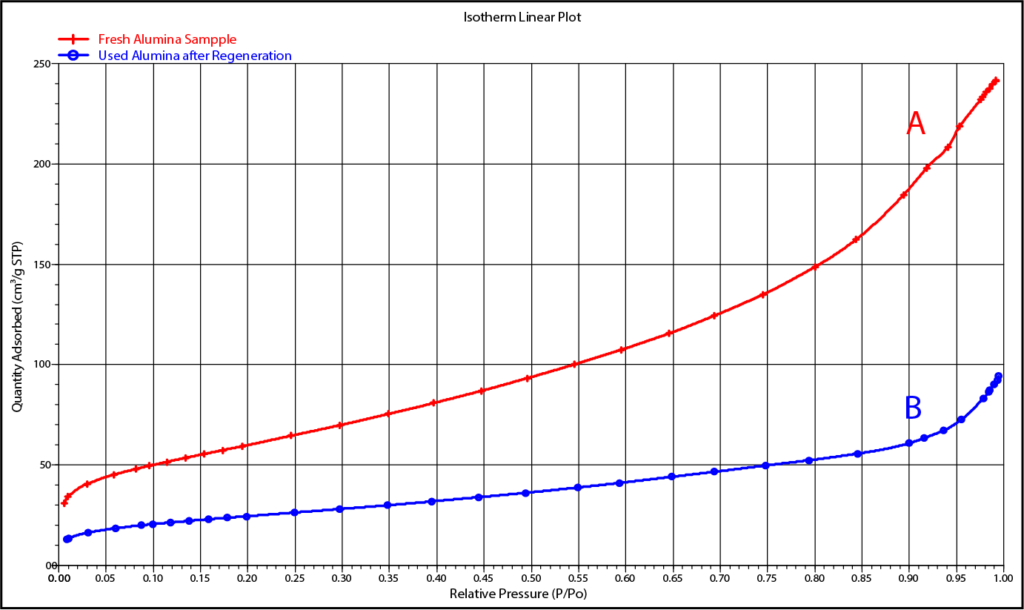
The isotherms in Figure 1 for fresh alumina (A) and for used alumina (B) show that the adsorption capacity of the fresh alumina reduces significantly with use.
The surface area of the sample, however, can be simply and quickly determined by the BET method using the data collected between 0.05 and 0.2 relative pressure. The value for the BET surface area is calculated using values determined for the gradient and intercept of the BET transformation plot together with the sample mass and well-defined physical constants, as shown in this example report.

Subsequent surface area results (Figure 3) can be compared to the values for the virgin material (Figure 2) and an objective judgment made as to whether to reuse the alumina or to replace it. In this case, analyzing the used sample by the BET method tells us that the available area has decreased by more than half and, therefore, the alumina should likely be replaced.
