Introduction
Breakthrough adsorption offers many advantages over static adsorption measurements. Multicomponent breakthrough measurements are performed by flowing multiple gas or vapor streams. A mass spectrometer measures the outlet concentration of the breakthrough column. The data is then assessed to determine the adsorption and separation performance of a material. Multicomponent measurements can be analyzed using a mass spectrometer for a large variety of gases and vapors. For isomeric compounds, that have overlapping mass spectrums, an FTIR is the preferred instrument for analyzing the outlet concentration of a breakthrough system.
In this note, single and multicomponent vapor mixtures are analyzed for zeolite 13X on the Micromeritics BreakThrough Analyzer (BTA). Vapor flows of water and ethanol were analyzed independently, masses 18 and 46 respectively, using a mass spectrometer. These measurements were conducted at 50 °C using equimolar carrier gas flowrates of nitrogen and helium.
The breakthrough system generates vapor streams by flowing an inert gas through a bubbler. Bubblers have been shown to be very efficient at generating saturated vapor streams at low flowrates. All components of the breakthrough system are housed within a hotbox, this prevent condensation of vapor streams and allows for a constant temperature throughout the analysis. Water and ethanol at 50 °C in an inert carrier gas will generate a saturated stream at the following vapor pressures: water 0.124 bar and ethanol 0.292 bar.
Experimental
Three vapor adsorption experiments were collected on zeolite 13X using water, ethanol, and a mixture of water and ethanol. Nitrogen and helium were used as the carrier gas and tracer gas for adsorption measurements as both are inert. Prior to analysis, the sample was activated at 100 °C for one hour and 200 °C for an additional 12 hours under nitrogen gas flow to remove any adsorbed species.
First, water vapor analysis was conducted at 50% RH using a split stream of dry nitrogen and humidified helium. The experiment was conducted at a pressure of 1.0 bar and a temperature of 50 °C. A total flowrate of 24 sccm was used for this experiment, 12 sccm was humidified helium and 12 sccm was dry nitrogen. The vapor pressure of water at this concentration is 0.124 bar.
Next, ethanol vapor analysis was conducted once again using a split stream of dry nitrogen and humidified helium. The experiment was conducted at a pressure of 1.0 bar and a temperature of 50 °C. A total flowrate of 24 sccm was used consisting of 12 sccm ethanol saturated helium and 12 sccm dry nitrogen. The vapor pressure of ethanol at this concentration is 0.292 bar.
A final experiment was conducting using mixed streams of ethanol and water vapor. The experiment was conducted at a pressure of 1.0 bar and a temperature of 50 °C. A total flowrate of 24 sccm was used consisting of 12 sccm water saturated nitrogen and 12 sccm ethanol saturated helium. The vapor pressures of water and ethanol are the same as those listed previously.
Results
Water
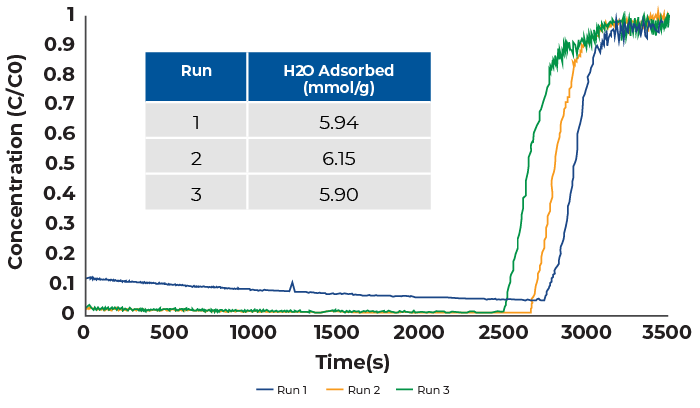
Water vapor breakthrough measurements were conducted by mixing a dry nitrogen stream with a water saturated helium stream in a one-to-one ratio. The total flowrate was 24 sccm which was comprised of 12 sccm nitrogen and 12 sccm helium. The breakthrough results are displayed in Figure 1.
The water breakthrough curve shows that there is substantial adsorption of water by zeolite 13X even though the concentration is low (vapor pressure is 0.124 bar). Breakthrough occurred roughly 45 minutes into the experiment for all measurements and the resulting breakthrough curves were sharp such that mass transfer limitations are minimal. Between each run the zeolite 13X sample was reactivated overnight at 200 °C. This step is required as water adsorbs strongly in zeolite 13X and was required for complete reactivation. Additionally, water is difficult to remove from mass spectrometers, prior to each measurement the mass spectrometer was baked out for several hours to drive water from the atmosphere out of the system.
Ethanol
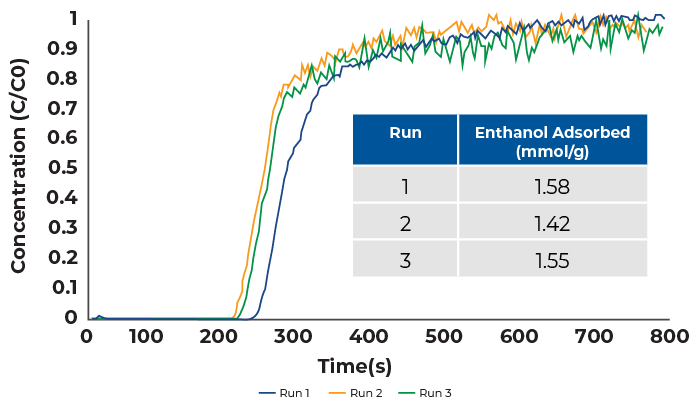
Ethanol vapor breakthrough experiments were conducted by mixing a dry nitrogen stream with an ethanol saturated helium stream in a ratio of one to one. The total flowrate was 24 sccm which was comprised of 12 sccm nitrogen and 12 sccm helium. The breakthrough results are displayed in Figure 2.
Subsequent ethanol breakthrough measurements were first collected without heated reactivation overnight. A pure stream of nitrogen was passed through the column for two hours, however a substantial drop-off in ethanol adsorption capacity was. Second pass capacity dropped to 0.49 mmol/g. For the tests show here, zeolite 13X was reactivated overnight at 200 °C for all measurements.
The ethanol breakthrough curves show that there is minimal drop-off in adsorption capacity between runs. During run one, 1.58 mmol/g ethanol was adsorbed compared to 1.42 mmol/g in run 2 and 1.55 mmol/g in run 3. All breakthrough curves are steep signifying that mass transfer limitations were not a concern during these analyses.
Ethanol-Water
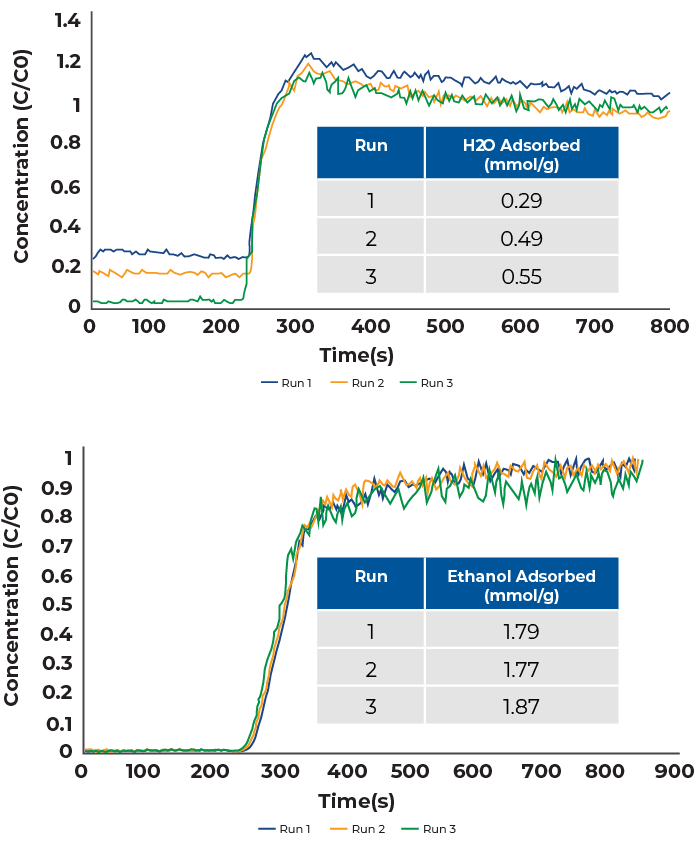
Ethanol-Water breakthrough experiments were conducted by mixing a water saturated helium stream with an ethanol saturated nitrogen stream. The total flowrate was 24 sccm which consisted of 12 sccm nitrogen and 12 sccm helium. While the flowrates of nitrogen and helium were the same, the flowrates of ethanol and water differ as water has a lower vapor pressure than ethanol at 50 °C. The breakthrough results are shown in Figure 3.
Between each measurement, samples were reactivated overnight at 200 °C to remove all adsorbed ethanol and water. Prior to adsorption measurements, the mass spectrometer was also baked-out to remove and water and ethanol remaining in the mass spectrometer.
The water and ethanol breakthrough curves display typical competitive adsorption behavior. Additionally, water and ethanol are partially miscible such that there is likely a synergistic effect in their adsorption behavior. Water vapor breaks through slightly before ethanol and displays slight roll-up before reaching saturation. The ethanol breakthrough curves look similar to those that were shown in Figure 2, however the adsorption capacity has been slightly increased. As mentioned before, this is likely due to a synergistic effect of combined water-ethanol adsorption. The water vapor adsorption capacity has been significantly suppressed compared to previous measurements and is further decreased due to preferential adsorption of ethanol. The preferential adsorption of ethanol is partially due to its increased concentration due to having a higher vapor pressure at 50 °C.
Conclusions
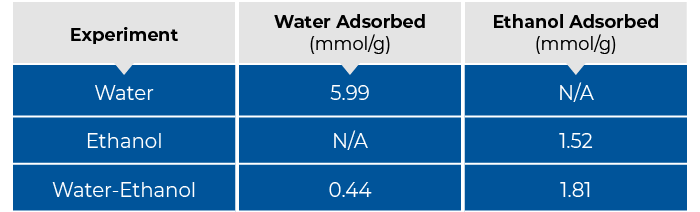
The app note showed pure component water and ethanol adsorption in zeolite 13X as well as competitive adsorption of these two species. Table 1 below summarizes the adsorption capacities of all three measurements. In the multicomponent water-ethanol breakthrough experiment, preferential adsorption of ethanol was observed. Across all measurements, no pressure drop was observed. Additionally, breakthrough curves were steep such that mass transfer limitations were insignificant.