Introduction
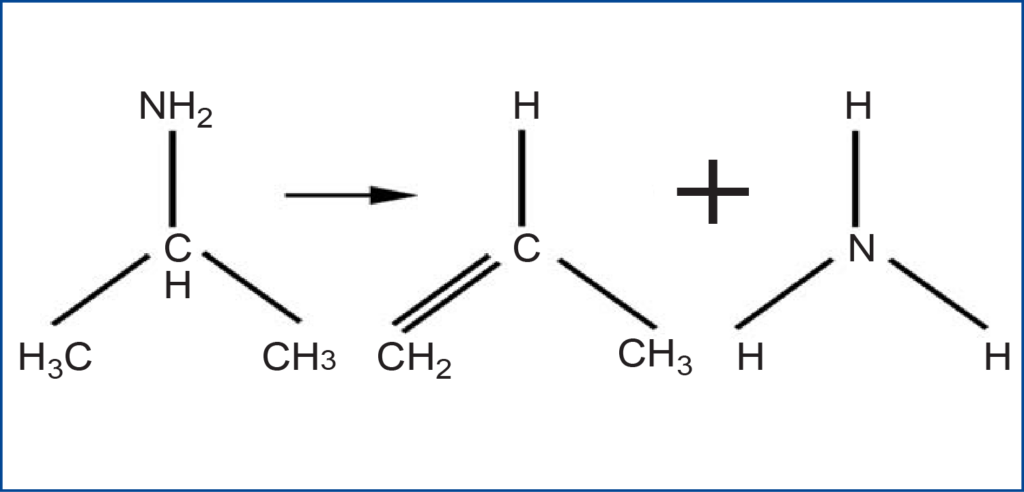
Several ZSM-5 zeolite samples were analyzed using the AutoChem II 2920. By pulsing isopropyl amine onto the zeolites, the concentration of acid sites can be determined for a particular zeolite. This is very important for catalytic reactions. For example, the reaction that occurred during these analyses proceeded through an intermediate due to an acid-base reaction between the amine and the zeolite, followed by a specific type of E2 elimination, known as the Hoffman elimination. Due to the second order kinetics involved in this reaction, a high concentration of acid sites would allow the reaction to proceed much more quickly.
Materials
The samples used are listed below.
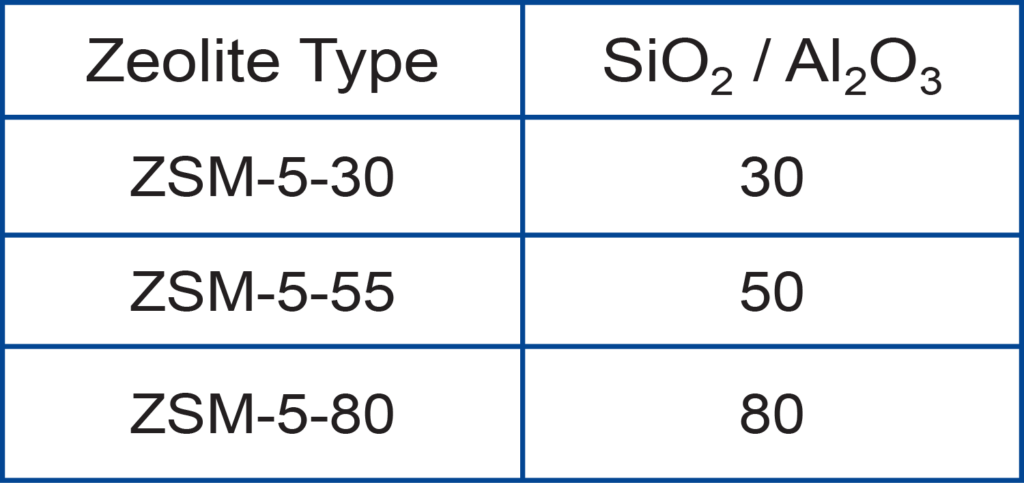
Preparation
While there was no preparation of the sample as far as degassing is concerned, it could be said that the sample was ‘prepared’ by heating. The ZSM-5 samples contained various cations, including ammonium (NH4+). These cations were not as desirable for the analysis as the hydrogen cation (H+). Since samples containing the hydrogen cation are more expensive, the sample can be converted to the hydrogen cation by heating the sample to high temperature. These samples were ‘prepared’ by first being heated to 500 °C and then cooling back to 200 °C.
Analysis
The preparation part of the analysis was followed by the pulse chemisorption step of the analysis. During this step, there The Hoffman elimination reaction: were twenty loop injections of isopropylamine occurring at four-minute intervals. The last part of the analysis involved a temperature-programmed desorption (TPD). At this step in the analysis, the mass spectrometer began scanning for propylene, the product of interest. Data were collected during a temperature ramp to 500 °C.
Data
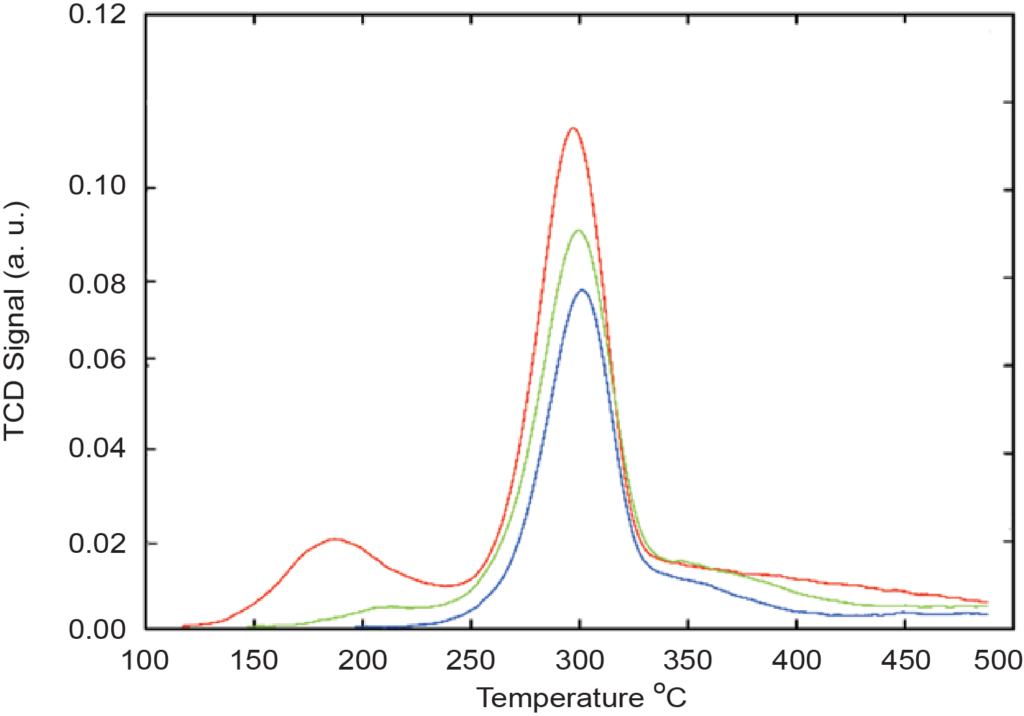
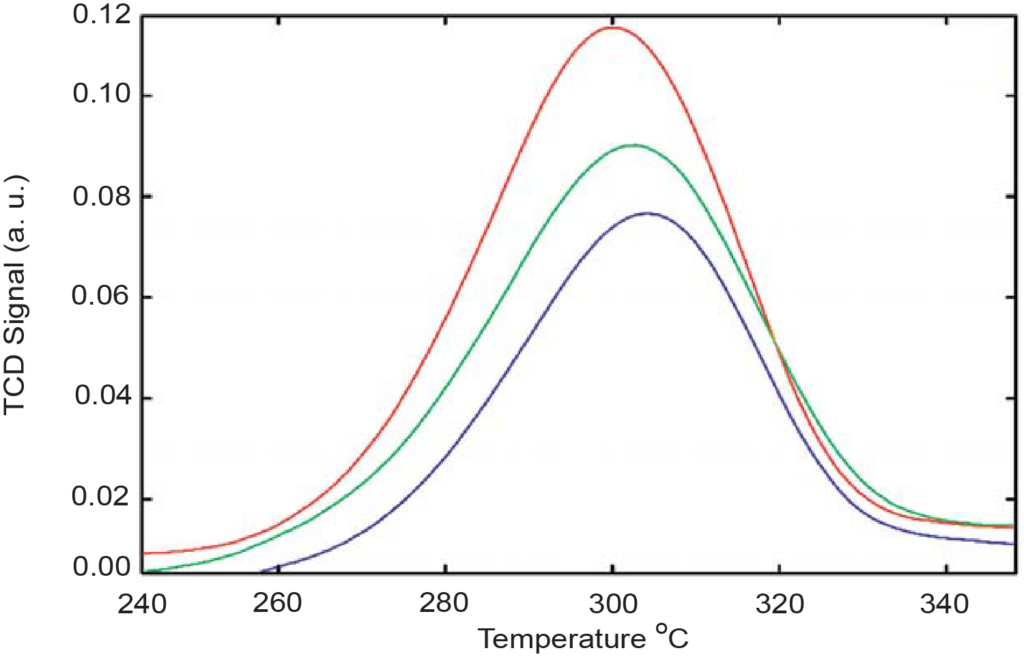
These graphs show how the intensity of the TCD signal changes for various zeolite samples. Notice that a direct proportionality exists between the peak intensities and the concentration of aluminum oxide (Al2O3), and an inverse proportionality exists between the peak intensity and the silica-to-aluminum oxide ratio (SiO2/Al2O3).