Many medicinal tablets are coated to improve in-vivo performance. Coatings are used to mask the taste of active pharmaceutical ingredients (APIs) and to control the location and rate of dissolution, to aid uptake, reduce side effects or prolong action via controlled release. As a result, coating integrity and thickness may be Critical Quality Attributes (CQA) for tablets. A coating must consistently cover the whole tablet and be sufficiently thick to transport the API safely to the intended site of action, but not so thick that the tablet passes through the site of action without dissolving. Being able to rapidly and reliably measure and control coating integrity is therefore crucial.
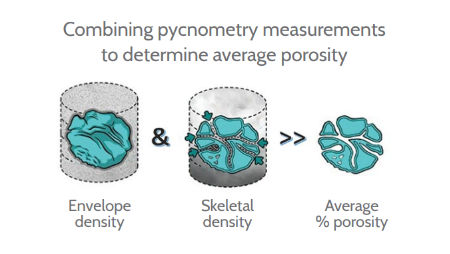
This application note describes a study in which the Micromeritics® AccuPyc II gas pycnometer and the Micromeritics® GeoPyc envelope density analyzer were used to compare the characteristics of coated and uncoated tablets. These instruments measure skeletal volume and envelope volume, respectively, this data combined with a supplied sample mass can generate four parameters, which are: skeletal and envelope density and the sample’s porosity. The porosity value can also be used to determine solid fraction and is simply [(100 – porosity) / 100]. This terminology is discussed further in the “Physical Properties Primer” below.
The results from this study highlight how skeletal density and porosity are impacted by scratching and splitting tablets, and how they can be used to assess coating quality. The data illustrate the potential and value of using these two measurements for monitoring coating processes.
The Equipment
The AccuPyc Gas Pycnometer
The AccuPyc is a gas pycnometer, it works by having two chambers, a sample chamber, and an expansion chamber, both with carefully calibrated volumes. A sample is loaded into the sample chamber and pressurized through valve a, the equilibrated pressure is then recorded using the transducer t. The gas is then expanded through valve b to the expansion chamber where the equilibrated pressure is again recorded. Using the ideal gas law, the volume occupied by the sample can be calculated from the known volume of the chambers and measured pressure values. The volume measured is the skeletal volume of the sample, since gas penetrates all accessible pore space. A more complete description of this is available in our application note 180 “Measuring the volume, density and porosity of tablets for coating process control and QC”.

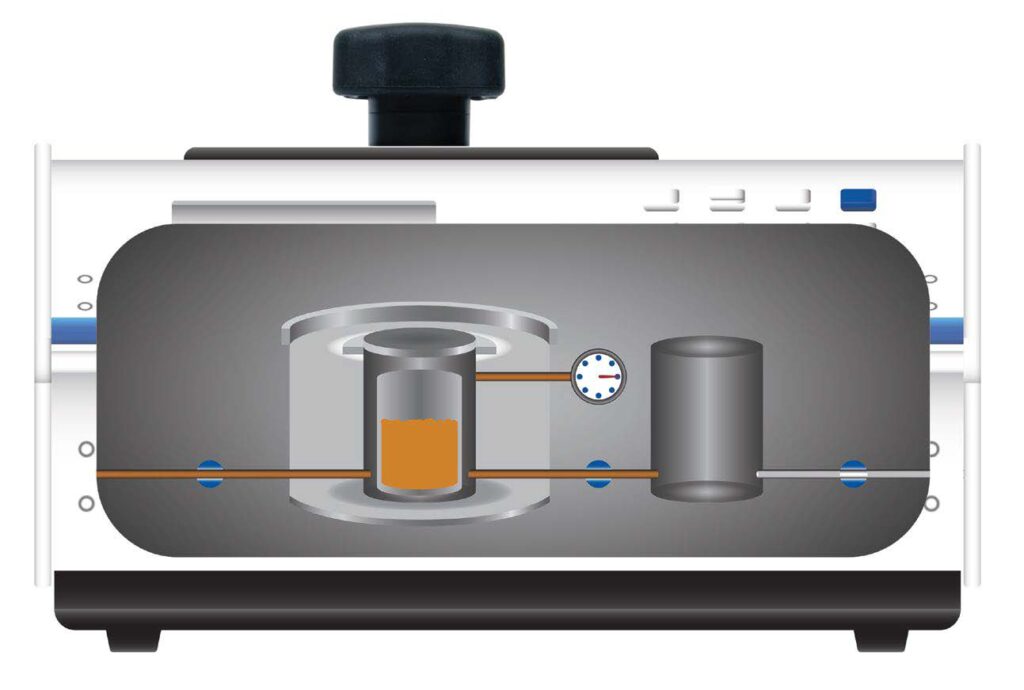
The AccuPyc measures skeletal volume by gas pycnometry
The GeoPyc Envelope Density Analyzer
The GeoPyc uses a precision cylinder of known diameter which is filled with a free-flowing quasi-fluid solid displacement media to enable measurement of envelope volume.
The cell is oscillated, to vibrate the media, which is simultaneously compressed to a defined consolidation force to establish the media volume (position A, in the Step 1: Blank). The sample is then added into the cylinder and the compaction process is repeated (position B, in the Step 2: Measurement).
The volume of the sample is calculated from the difference in the distance that the piston travels to achieve an equivalent consolidation force (h, the distance between positions A and B, in the Step 2: Measurement box).
The Micromeritics® DryFlow displacement media conforms to the surface of the sample during measurement but does not penetrate any pore space. The media forms a tightly compacted layer around the sample regardless of the sample’s geometry. The resulting volume is therefore described as the envelope volume.

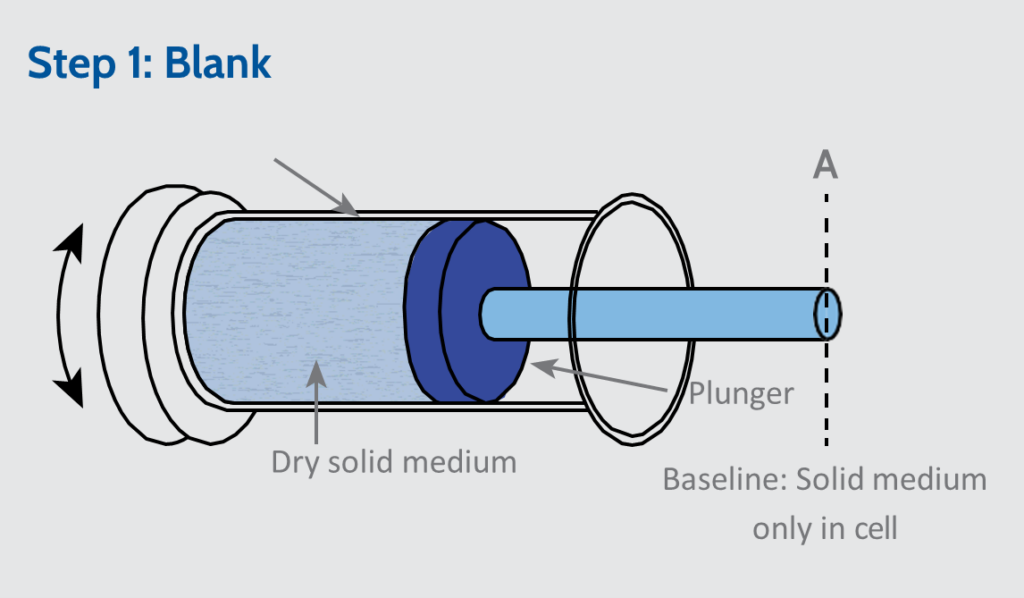

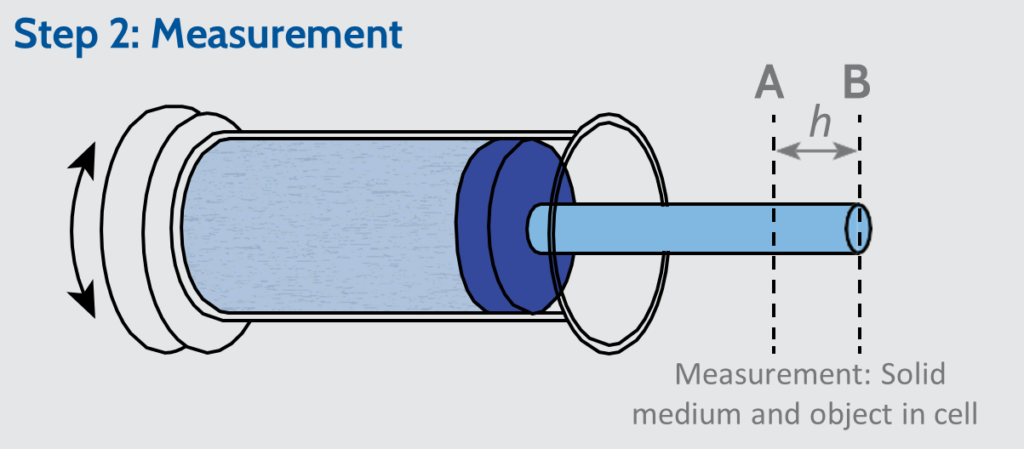
The GeoPyc measures envelope volume by solid phase displacement
Experimental Details
Samples of indigestion and enterically coated Aspirin tablets were tested using the AccuPyc and GeoPyc. The indigestion tablets are uncoated, designed to be chewed in the mouth and swallowed. They react and dissolve in the acid of the stomach. In contrast, the Aspirin tablets are polymer coated to allow them to pass through the stomach intact. While the polymer does not react with the acid of the stomach, it rapidly breaks down in the more alkaline conditions of the small intestine, allowing targeted dissolution and API absorption.
The tablets were tested whole, scratched (to simulate damage to the coating) and halved. Thus, testing the ability of the systems to detect defects in the coating layer. Both types of tablets were treated in the same way to generate a comprehensive set of comparative data; where the uncoated tablets act as a control, and duplicate tests were performed for each sample.
All sample preparation and testing, including system calibrations, data collection for 12 sample sets and bookend tests was completed in around 4.5 hours.
Full details of the experimental methods, instrument settings used, and data processing steps applied can be found in separate white paper. Please contact us for additional information on the white paper.

Results and Data Analysis
Difference plots for the two sets of tablets, showing skeletal density and porosity are given below. These two parameters exhibited the most change, from sample to sample. The difference values were produced by subtracting the lowest value of each dataset, the value for the whole tablet in each case, from each of the other values.
Looking first at the data for the change in skeletal density (left graph), it is clear that scratching or halving has a much greater impact on the coated Aspirin tablet (turquoise bars) than the uncoated indigestion tablet (green). With the indigestion tablet, the response is roughly double for halved as compared to scratched, but in absolute terms the change observed is approximately comparable to the error associated with the measurements. The statistical significance of this trend is, therefore, weak. In contrast, scratching of the Aspirin results in a significant change in density, with further damage (halving) only giving rise to a modest further change. These results suggest that change in skeletal density may be a sensitive indicator of damage to the tablet coating.
The porosity data for the coated Aspirin (right graph – purple bars) shows a more linear trend, from whole to scratched to halved, is seen. This may also be sufficiently sensitive to detect coating damage. These data suggest that the tablet becomes more porous, once the interior, uncoated material is exposed, an expected result. The results for the indigestion tablet (blue bars) shows a more marked trend than expected, particularly given the density data. They suggest that the tablet is not homogeneous, but rather that the outer surfaces of the tablet are denser and less porous than the center, possibly as a result of non-uniform compaction in the tablet press. Taken together, the two sets of data indicate that while porosity could be used to detect an issue with coating integrity, it is a less useful parameter than density, since both coated and uncoated tablets show the same trend.
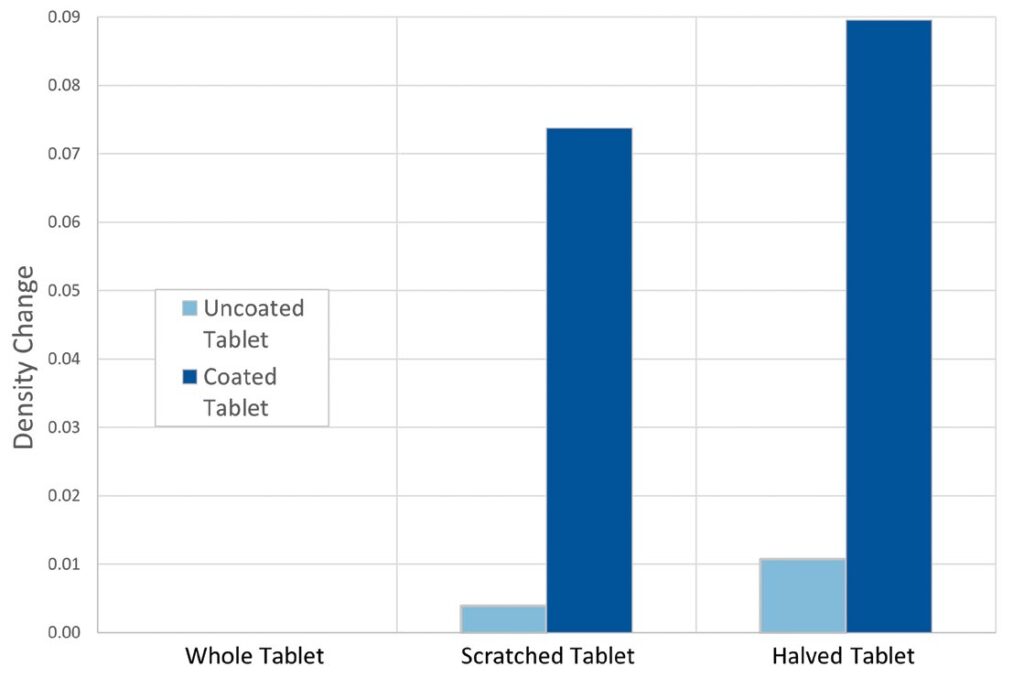
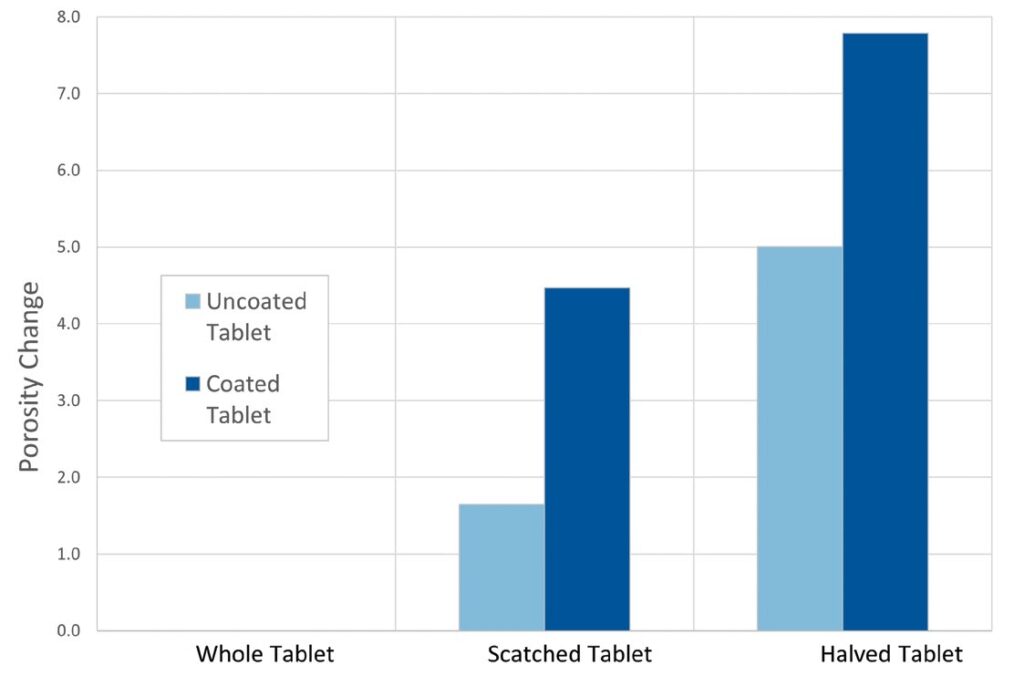
Difference plots of density or porosity change for both tablet types versus damage to tablet. Showing the impact of scratching and halving is more marked for the coated Aspirin tablets
Conclusion
This study highlights the value of the AccuPyc and GeoPyc to be used in combination for the fast and simple monitoring of the coating integrity of tablets for process control or in QC.
Together the AccuPyc and GeoPyc enable the rapid and reliable measurement of five physical characteristics, all of which may be exploited for pharmaceutical process control.
The data presented here clearly show that:
- Both porosity and skeletal density change as a result of loss of integrity of a tablet coating
- The density results are sufficiently discriminating to detect even relatively minor surface defects
Physical Properties Primer – Density, Volume and Porosity
Density
At first sight, density is a relatively straight forward term defined as mass divided by volume. On a lab scale, values are typically stated in g/cm3 . However, there are multiple ways to define and quantify volume. Each gives rise to a different density parameter.
Volume
In the study described here, envelope and skeletal density were determined from measurements of:
- Envelope volume, which is the volume the sample occupies in space, including both the solid content of the sample and any pore space or voids within it
- Skeletal volume, which is the volume of actual solid that makes up the sample
Porosity
Porosity is a dimensionless value, normally quoted as a percentage. It quantifies how much of a sample is solid and how much is empty space. Porosity can be calculated using the equation below when working with volume values and is independent of mass. There is also a corresponding equation that can be used with density values, as porosity is independent of mass the volume equation is preferred here.
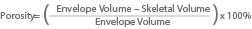
The solid fraction can also be calculated from a knowledge of porosity and is given by the equation:
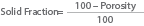
These values are interrelated where porosity describes the amount of empty space and solid fraction describes the amount of solid in any given sample. Therefore, a high value for solid fraction indicates a high solid content in the sample, and this would correspond to a low porosity. Conversely, a high value for porosity indicates a high total pore volume in the sample, and would correspond to a low solid fraction value.