Determination of the dispersion of active species on catalysts is a tremendous tool to discover and predict the activity of the catalyst. Thus, dispersion of active species indicate the quantity of active particles located on the surface of a catalyst that are accessible and have a direct contact with the reactant molecules that have to react and produce a new substance. Hence, a correct measurement of the dispersion of the active species predict the activity of the catalyst for a specific catalytic process.
The technique comprises the reduction of the active particles in the catalyst at elevated temperature. Usually Hydrogen is widely used for this task and that could be used as pure hydrogen or a mixture of H2 balanced inert gas. Upon completion of reduction and the catalyst temperature is brought back to room temperature, the catalyst is then titrated by using a calibrated loop and dosing known amount of active gas, usually is carbon monoxide or hydrogen. Therefore and upon saturation, the amount of adsorbed active gas is being calculated and related to the accessible active species on the surface of the catalyst. This method of titration is by far, the most useful tool to predict the activity of the catalyst.
The problem arises during the removal of the remaining hydrogen on the catalyst after reduction. This task is usually done by flowing an inert gas over the sample at the same reduction temperature. Removal of the remaining H2 can take some time, could take one hour or sometimes more than that depending of the active particles themselves and their ability to retain H2. Upon removal of the remaining H2, the sample temperature is brought back to room temperature having the inert gas still flowing over the sample. If the inert gas being used, contains traces of O2, will slightly oxidize the freshly produced reduced particles, and hence, change the composition of the accessible particles on the surface that used to determine the dispersion.
Experimental
A 0.5% Pt/Alumina Micromeritics reference material with a 35 percent dispersion plus or minus 5, was considered for the experiments. The sample was first reduced by flowing a 100 ml/min of hydrogen at 400C for one hour. Upon reduction, sample was swept by 100 ml/min of Helium at the reduction temperature for 30 minutes. Then the sample temperature was reduced to room temperature at which pulse of active gas was carried out until saturation. A mass spectrometer Cirrus II was used as a detector to follow the masses involved in the analysis.
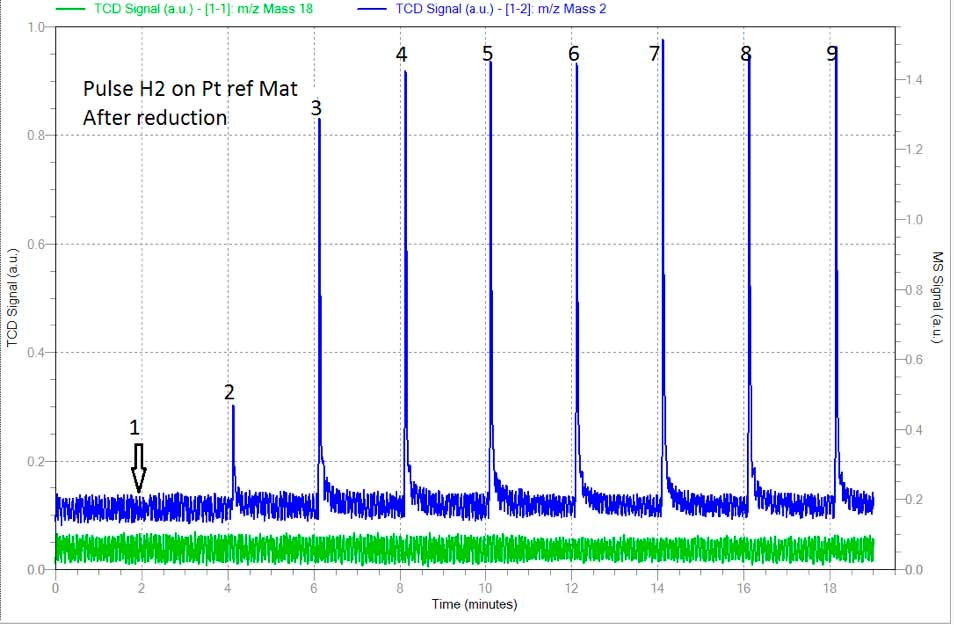
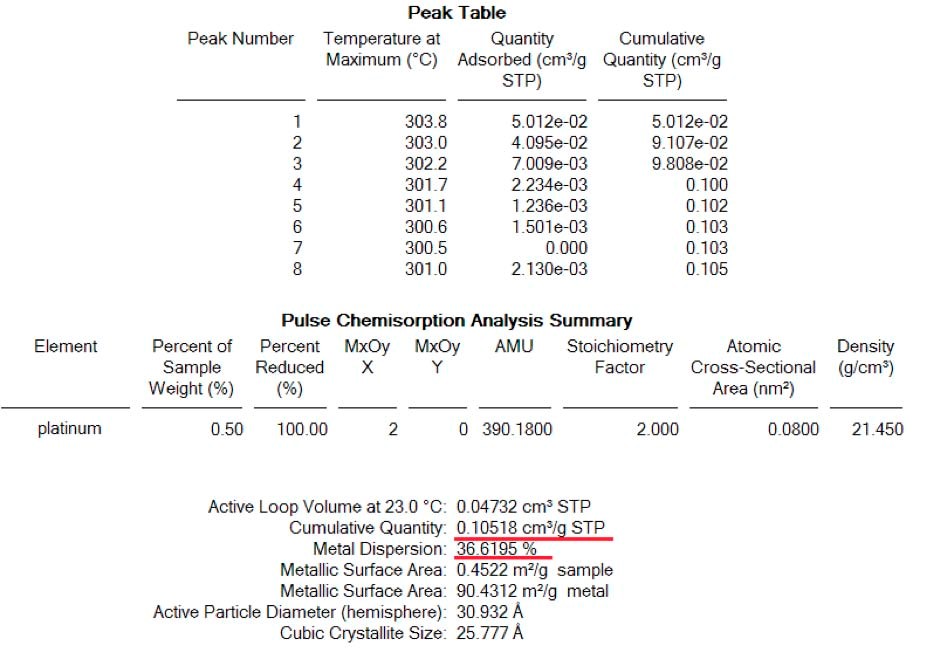
The first analysis was carried out after reduction and a complete removal of the remaining hydrogen. Pulse of 0.0513 ml were carried out and signal of mass 2 (H2) was followed by mass spectrometer to ensure complete saturation of the sample. Figure 1 shows the spectrum of H2 where one pulse was completely adsorbed while peaks 5 to 9 show complete saturation and were taken into account for the determination of the total amount of H2 adsorbed. (See table 1)
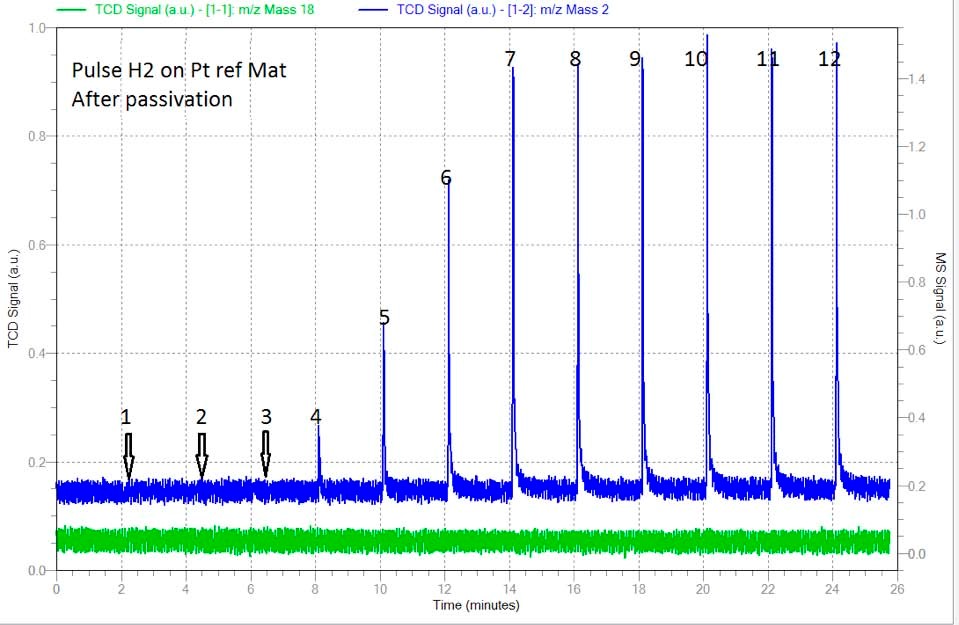
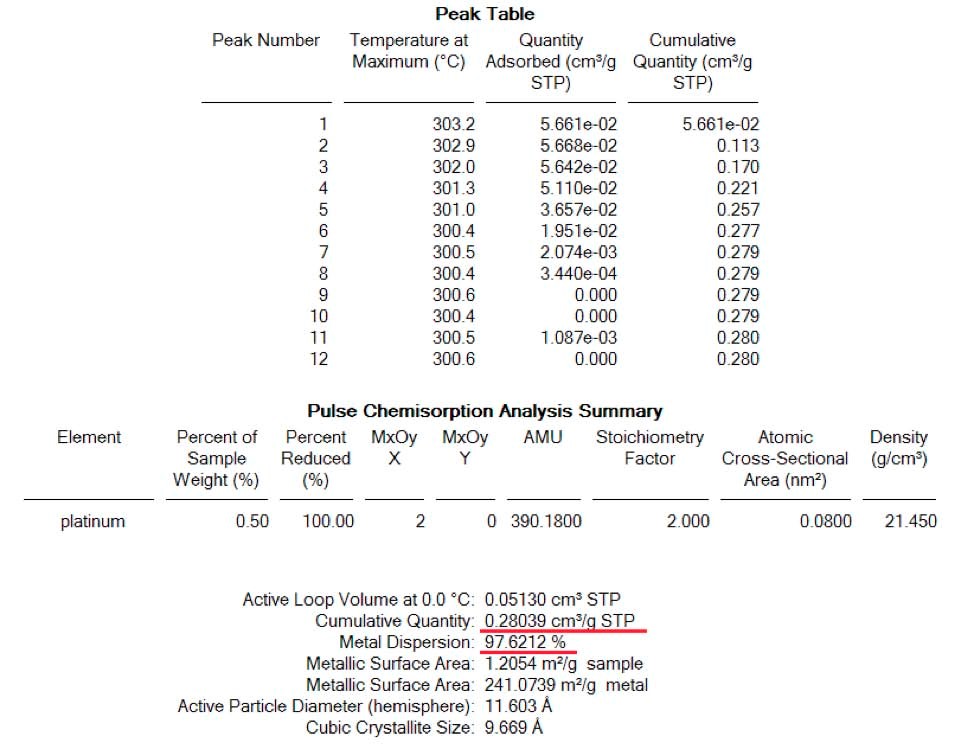
Following this first analysis, sample was taken back to 400C under the flow of 100 ml/min of helium to remove all adsorbed H2. A complete removal of H2 at 400C was followed by the signal of H2 (mass 2) on the Mass Spectrometer. Right after, the sample was brought to room temperature under the same flow of helium. At this step, a 0.1 ml of air (approximately 0.03 ml of oxygen) was injected using a syringe to the carrier gas in order to simulate the presence of traces of O2 in case of the use of a contaminated Helium in order to see its effect on the dispersion. Figure 2 shows for the same pulse technique, larger amount of H2 being adsorbed by the sample. In this case, 4 full injections were completely adsorbed by the same sample shown in figure 1. This effect demonstrate that the presence of traces of O2 in the inert gas used to clean the sample from the remaining H2 after reduction, will badly alter the dispersion results. In this case the dispersion was inflated by a factor of 3 approximately. (See table 2)
The same procedure described above was repeated but Carbon Monoxide was used as active gas instead of H2.
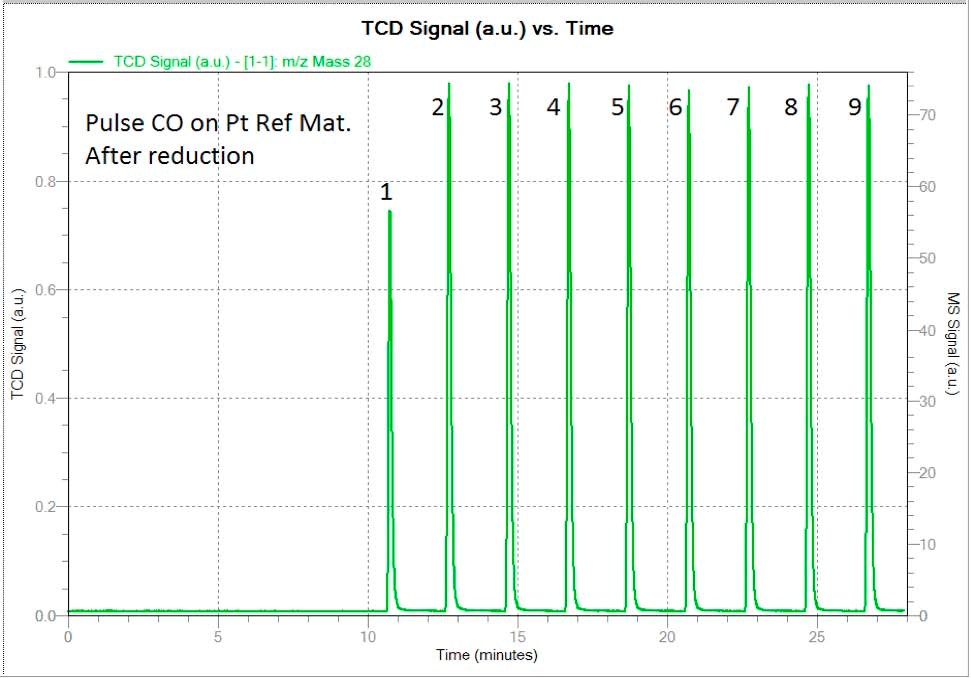
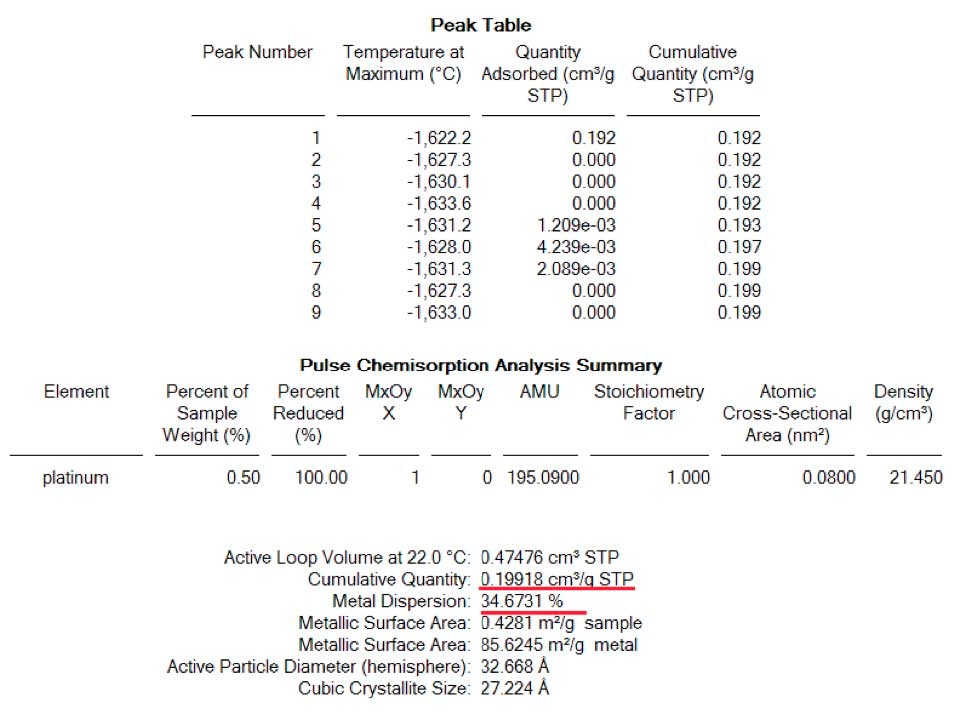
First, the analysis was carried out without the passivation procedure described above. Result of CO chemisorption indicated on figure 3, yield the correct value of the dispersion that is 35 percent plus or minus 5. (See table 3)
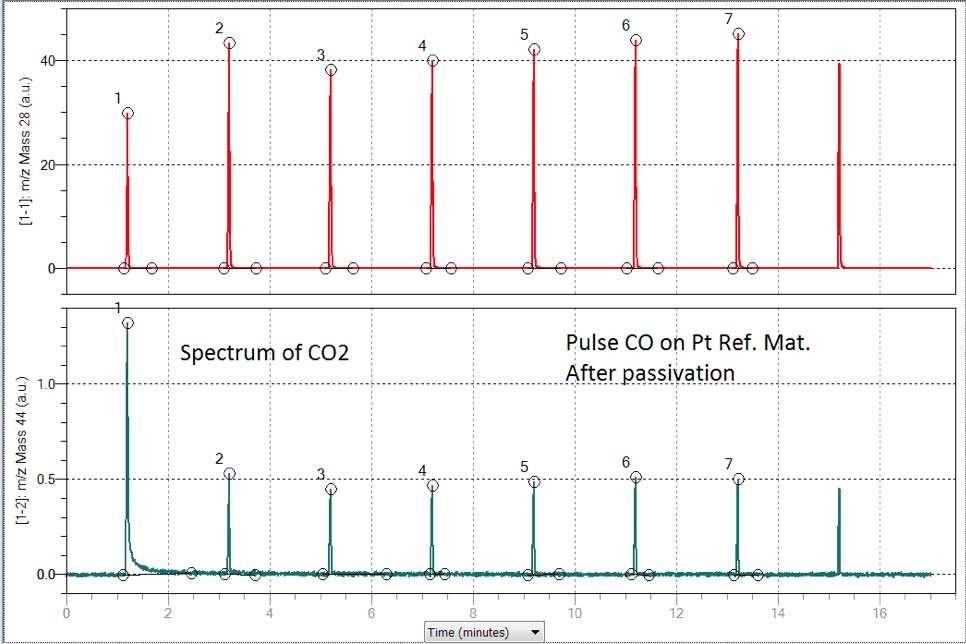
Figure 4 shows two spectra corresponding to the CO pulse chemisorption. The above one demonstrates the spectrum of CO coming from the passivated sample as was done in the case above for hydrogen. A 1/3 of the first pulse was adsorbed while the rest of the peaks indicate saturation.
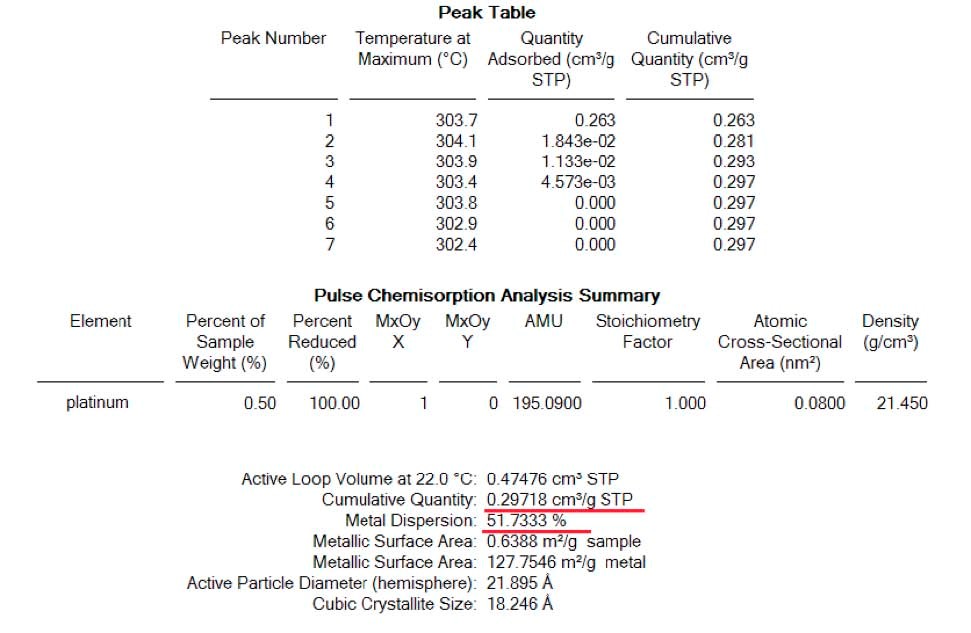
The below spectrum of figure 4 correspond to mass 44 at indicates the formation of Carbon dioxide while CO being pulsed on an oxidized or passivated sample. The final result of dispersion indicates that the presence of traces of O2 is responsible for the overestimation of the dispersion on the Micromeritics reference material. (see table 4)
Results of Hydrogen Chemisorption:
Result of quantities adsorbed as well as dispersion for each analysis are shown here below:
Results obtained by CO chemisorption:
Conclusion
It can be concluded from this work that chemisorption technique is highly sensitive to contamination, especially for the inert gas that is used to remove the excess of hydrogen that remains adsorbed by the sample after reduction.
The overestimation of the result will depend on the quantity of contamination existing in the carrier gas. In any case, pure gases are required for a good chemisorption analysis, otherwise, results will not offer any meaning, especially if are related to the activity of the catalyst.
In the case of H2 chemisorption, a large amount of H2 is being adsorbed. One part is being adsorbed by the atoms of Platinum, while the large amount of H2 is being retained or adsorbed by the atoms of O2 located on the platinum atoms on the surface of the solid. However, it can be affirmed that molecules of H2 are only adsorbed without reaction as spectrum of water indicates the complete absence of it.
Results from CO chemisorption however, show somehow different results. Carbon Monoxide shows higher activity than H2 over atoms of Platinum, is capable to remove the O2 atoms and produce carbon dioxide as has been shown on figure 4 resulting in higher dispersion of the material, table 4.