by: Reid Davis
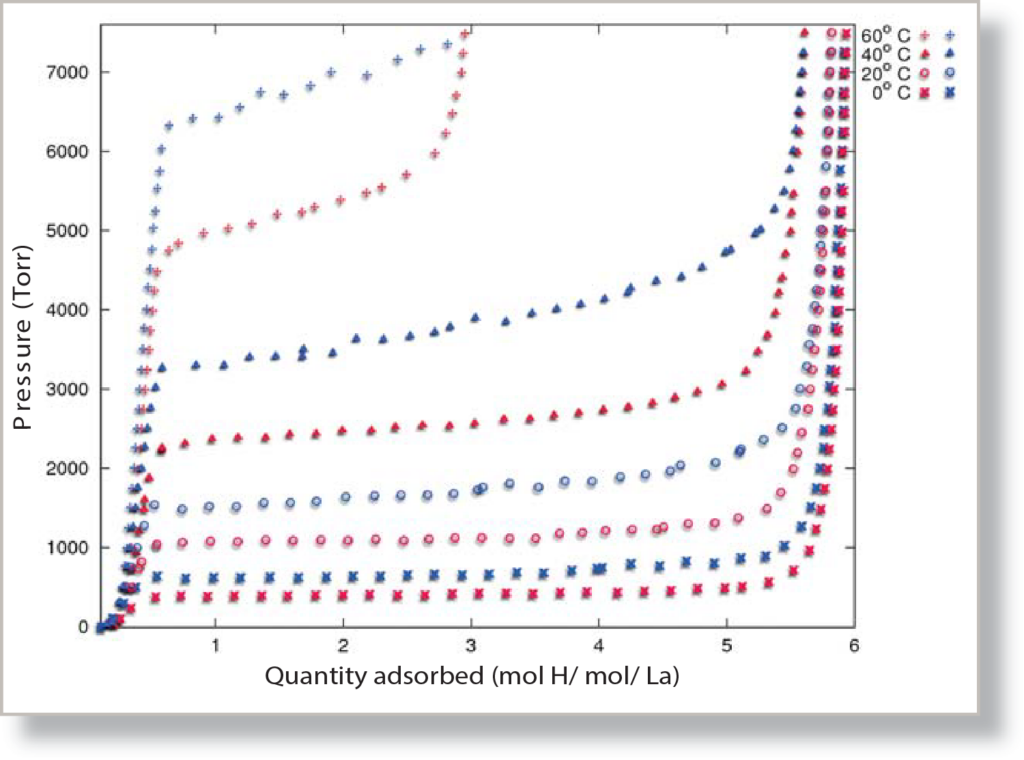
Lanthanum pentanickel (LaNi5) is a metal alloy that, under the correct pressure and temperature conditions, will rapidly sorb hydrogen gas and form a metal hydride compound. Hydride forming metal compounds are well known for their ability to sorb large amounts of hydrogen gas at a specific pressure, store it, and later release the hydrogen at a different, lower pressure. This sorption behavior leads to a distinct “plateau” when hydrogen reacts with the LaNi5, a reaction that is highly dependent on temperature, as demonstrated by the pressure composition isotherm, Figure 1. By using the isotherms collected at different temperatures, a heat of adsorption report can be generated, a report that gives some insight to the reaction mechanisms of the LaNi5-hydrogen system.
Materials
A finely powdered 99.9% pure LaNi5 was used for this analysis. The hydrogen sample was an ultra high purity (UHP) grade hydrogen, and likewise, UHP helium was used to measure free space after analyses. The LaNi5 used for analysis was acquired from Alfa Aesar®.
Preparation
The LaNi5 sample can be prepared by an extended soak in a high pressure, pure hydrogen environment. The sample used in these analyses was first soaked as a large batch in hydrogen at 250 psi (~13,000 Torr) for 24 hours, and then a smaller sample of five grams was again soaked at 150 psi (~7500 Torr) on the sample port of the ASAP 2050 for an additional 48 hours prior to running an analysis. The second soaking was necessary because the LaNi5 was possibly exposed to air during routine handling of the material. An additonal hydrogen soak is used to purify and reduce the LaNi5.
Analysis
After the LaNi5 had been soaked in pure hydrogen, several analyses were performed. An analysis consists of collecting a full pressure range isotherm on the ASAP 2050, from 0.1 Torr to 7500 Torr. Analyses were also run at a wide range of temperatures, from 0 oC up to 60 oC, with analyses run at intermediate temperatures of 20 and 40 oC.
Data
By running multiple analyses on the same sample at different temperatures, it becomes possible to generate an isosteric heat of adsorption report on the sample. The isosteric heat of adsorption is the amount of energy required for the adsorbate, the gas, to adsorb onto the adsorbent, the sample. The isosteric heat of adsorption is calculated using isotherms of the sample at multiple temperatures. The pressure is interpolated to a set of equally spaced volume increments. Using the interpolated pressure and volumes, the natural log of each pressure point for their respective volumes is plotted with respect to 1/RT. The heat of adsorption can be directly calculated for each isostere on plot by using a derivation of the van’t Hoff equation:
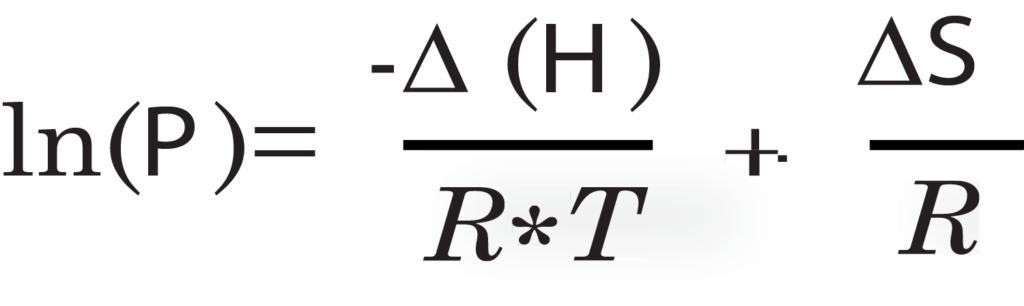
where:
<delta>H is enthalpy (heat) of of adsorption in kJ/mol, <delta>S is
entropy of sorption in kJ/mol*K, P is the pressure in Torr, and R is the gas constant, 0.0083144 kJ/mol*K.
Taking the slope of each isosteric line yields the enthalpy of adsorption for the specific volume that the isostere corresponds to, and from the volume and enthalpy data, the heat of adsorption plot can be created, which can be seen in Figure 2. The overall heat of adsorption for the LaNi5 sample can be compared to the average value of the plateau, which is 30.295 kJ/mol for hydrogen absorbing on LaNi5.
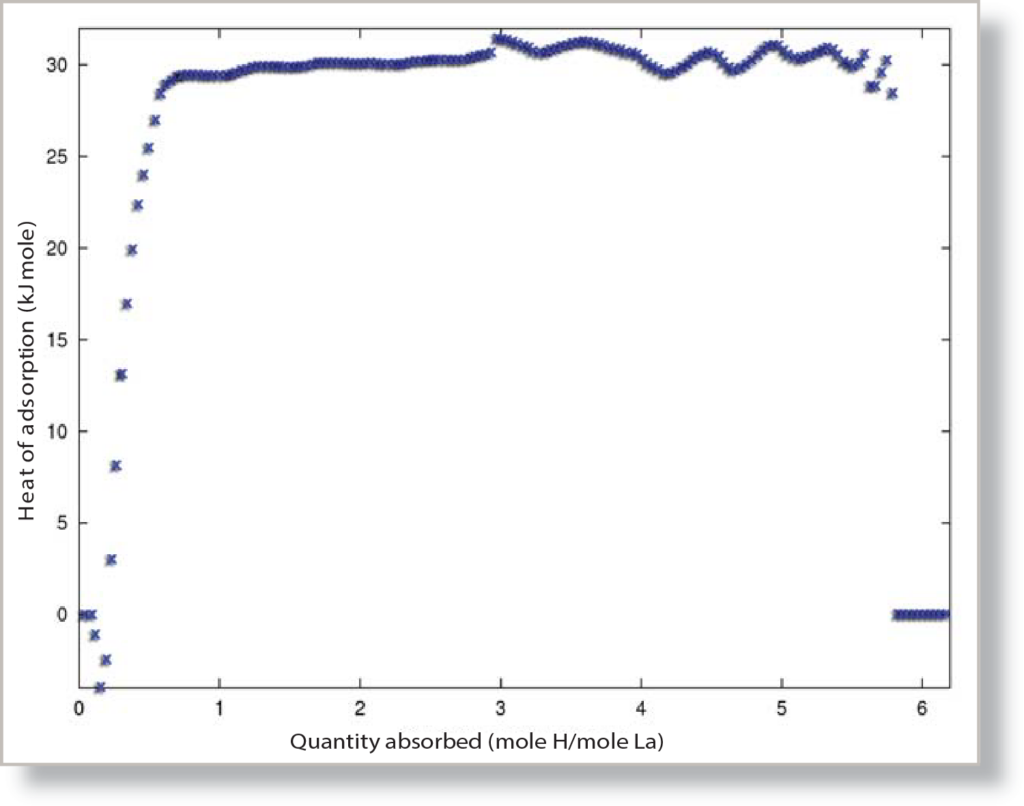
The heat of adsorption for hydrogen during physisorption is somewhere between 4 to 10 kJ/mol[1] for a typical carbon sample, but for the LaNi5 sample, the heat of adsorption is calculated to be 30.295 kJ/mol, agreeing with published data, which has LaNi5’s enthalpy ranging from about 29 to 32 kJ/ mol[2]. This increase in the heat of adsorption over typical enthalpies for the physisorption of hydrogen is the result of the LaNi5 disassociating and absorbing hydrogen. Unlike most materials run on the ASAP 2050, such as carbons, which employ physisorption to adsorb molecular hydrogen, the LaNi5 employs chemisorption and actually absorbs atomic hydrogen into the metal structure. During the chemisorption, the hydrogen molecule dissociates and absorbs into the LaNi5 as two hydrogen atoms: Because of the splitting of the bond in the hydrogen molecule, the heat of adsorption greatly increases, up to about 19.6 kJ/mol from the dissociation effects alone at 750 Torr and 300 K[3]. The chemisorption of the hydrogen has the strongest effect on the heat of adsorption, but the absorption of hydrogen also plays a significant role in the increased heat of sorption. The hydrogen atoms from the dissociation process absorb into interstitial sites in the metal lattice, causing the lattice to expand, contributing most of the remainder of the increased heat of adsorption[4]. By using the heat of adsorption report, it becomes apparent that much more than simple physisorption occurs for the hydrogen sorption and storage of LaNi5 and interpretation of the results can help determine the interaction between the hydrogen and the LaNi5.
References
- Gigras, A., Bhatia, S., Kumar, A., Myers, A.. Feasibility of tailoring for high isosteric heat to improve effectiveness of hydrogen storage in carbons. Carbon 45, 1043-1050
- Schlapbach, L., Züttel, A. Hydrogen-storage materials for mobile applications. Nature 414, 353-358
- Sandrock, G., Thomas, G. The IEA/DOE/SNL on-line hydride databases. Appl. Phys. A 72, 153-155
- Yamamoto, T., Inui, H., Yamaguchi, M. Effects of lattice defects on hydrogen adsorption-desorption pressures in LaNi5. Materials Science and Engineering 329-331, 367-371