Introduction
Isotherms are typically collected using static adsorption measurements as it is both easier and faster than collecting isothermal data on a breakthrough system. Static measurements, however, cannot mimic flows or gas impurities, and for this reason it may be necessary to collect breakthrough data at varying pressures to analyze and optimize your process. This application note will target how changing pressure can impact the quantity adsorbed by a material and how measuring breakthrough curves at different pressures would allow a user to construct an isotherm. Isotherms determined via breakthrough analysis will never correlate exactly with those that are produced using static adsorption measurements. The impact of flow can have a drastic effect on the adsorption isotherm, especially when pressure drop or mass transfer limitations are involved.
Experimental
Zeolite 13X was used to measure high pressure carbon dioxide adsorption on the Micromeritics BreakThrough Analyzer at varying pressures between 1 bar and 10 bar (absolute). These measurements were collected at 30 °C and a total flowrate of 21 sccm. The flow consisted of 10 sccm N2, 10 sccm CO2, and 1 sccm He. Helium was used as a tracer gas in these experiments to determine the start of the breakthrough experiment. A tracer gas was used as the total flowrate of gas remained the same regardless of pressure, however the deadtime of each experiment would change due to the compression of gas in the same volume of total dead space. Prior to each analysis, zeolite 13X was activated at 100 °C for one hour before increasing to 200 °C for an additional 12 hours. After each measurement, the sample required full activation to completely regenerate the material, while room temperature activation was sufficient at 1 bar, higher activation temperature were required for greater pressure measurements. Additionally, the mass spectrometer would become saturate with CO2 after each measurement and required a brief 1 – 2 hour bake-out before proceeding to the next analysis.
Results
Breakthrough measurements were collected on zeolite 13X at 1, 2, 3, 5, 7, and 10 bar absolute pressure. For each measurement the flowrate of CO2 was 10 sccm. Figure 1 shows the results of all breakthrough measurements. Time zero corresponds to the breakthrough of helium in the measurement such that the deadtime of the experiment has already been subtracted out.
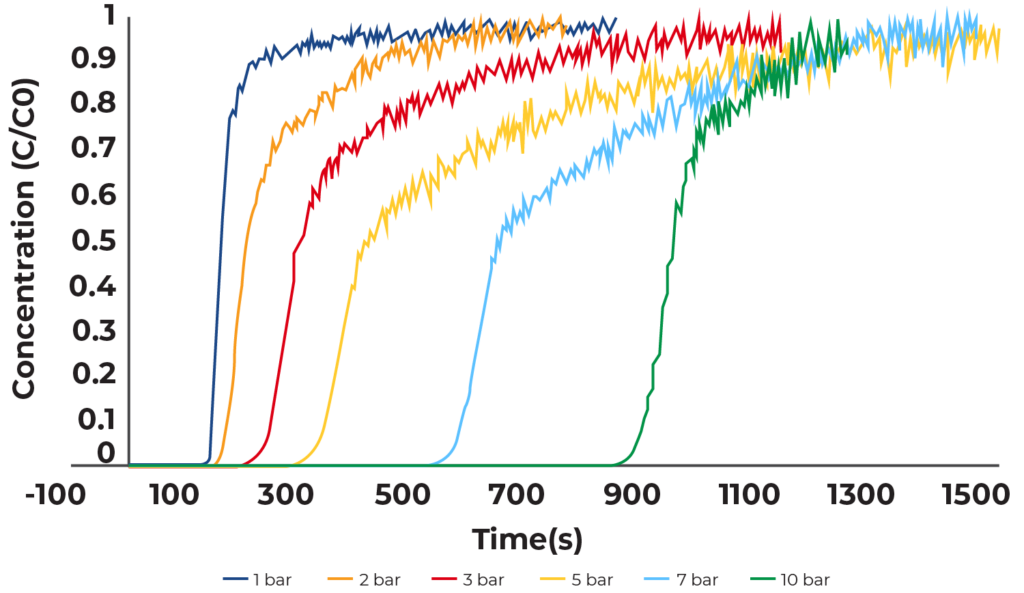
All the zeolite 13X breakthrough curves show sharp peaks signifying that there are little to no mass transfer limitations. This behavior is expected as zeolite 13X has shown excellent capabilities to adsorb CO2 and the pore window is much larger than the kinetic diameter of CO2. Additionally, no pressure drop was observed in the system, this was also expected as zeolite 13X was analyzed as-is in its pelletized form.
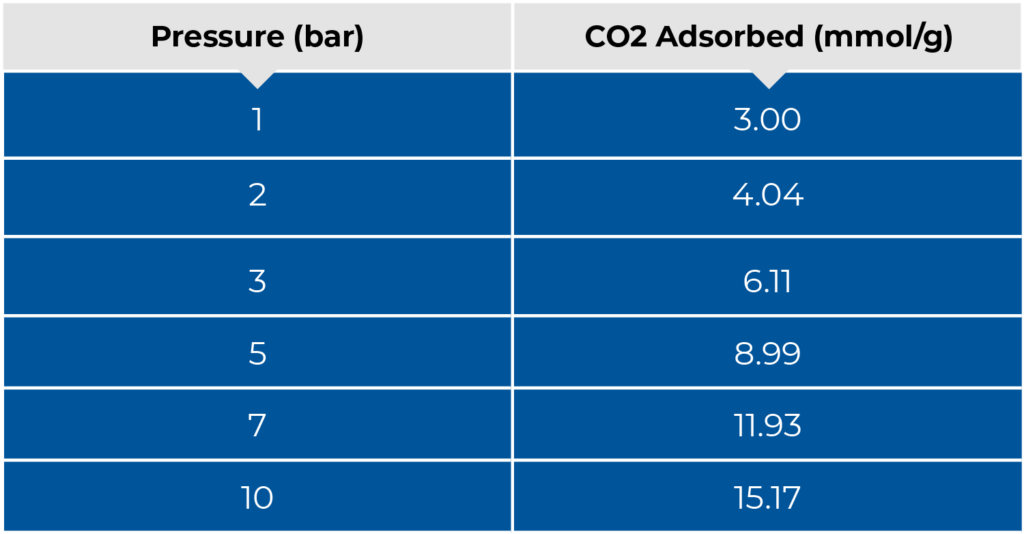
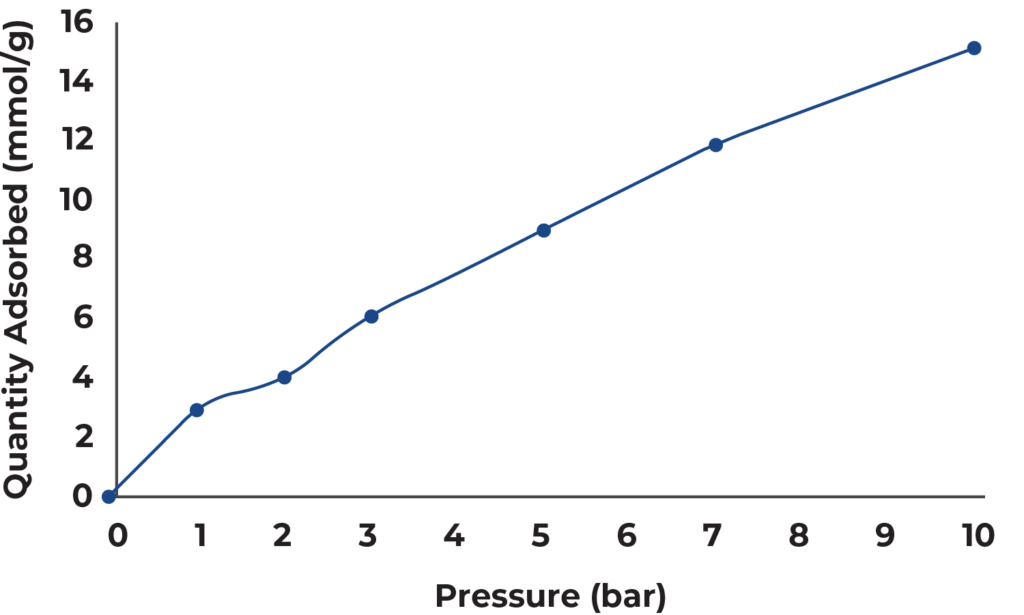
Figure 2 below shows the isotherm that is generated when solving the breakthrough equation and
plotting the total quantity adsorbed for each breakthrough measurement. Table 1 also displays the
quantities adsorbed at each pressure.
Conclusions
Zeolite 13X is an effective CO2 adsorbent at atmospheric and high pressures. Zeolite 13X displayed a CO2 adsorption capacity of 15 mmol/g at 10 bar total pressure. Throughout the breakthrough measurements, it showed little to no pressure drop and no mass transfer limitations as were seen in sharp breakthrough curves.